Gm-csf/cd40l vaccine and checkpoint inhibitor combination therapy
An immune checkpoint and inhibitor technology, applied in drug combination, immunoglobulin, antibody medical components, etc., can solve problems such as insufficient clinical efficacy
Inactive Publication Date: 2017-05-24
H LEE MOFFITT CANCER CENT & RES INST INC
View PDF10 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Therefore, a single treatment, such as immunization with a vaccine, may not be sufficient for clinical efficacy
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0069] Example 1: A randomized phase I / II clinical study of nivolumab combined with GM.CD40L vaccine for lung adenocarcinoma.
[0070] The following is a description of a Phase II clinical trial to establish the efficacy of combining anti-PD-1 therapy with a GM.CD40L tumor vaccine.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Login to View More
Abstract
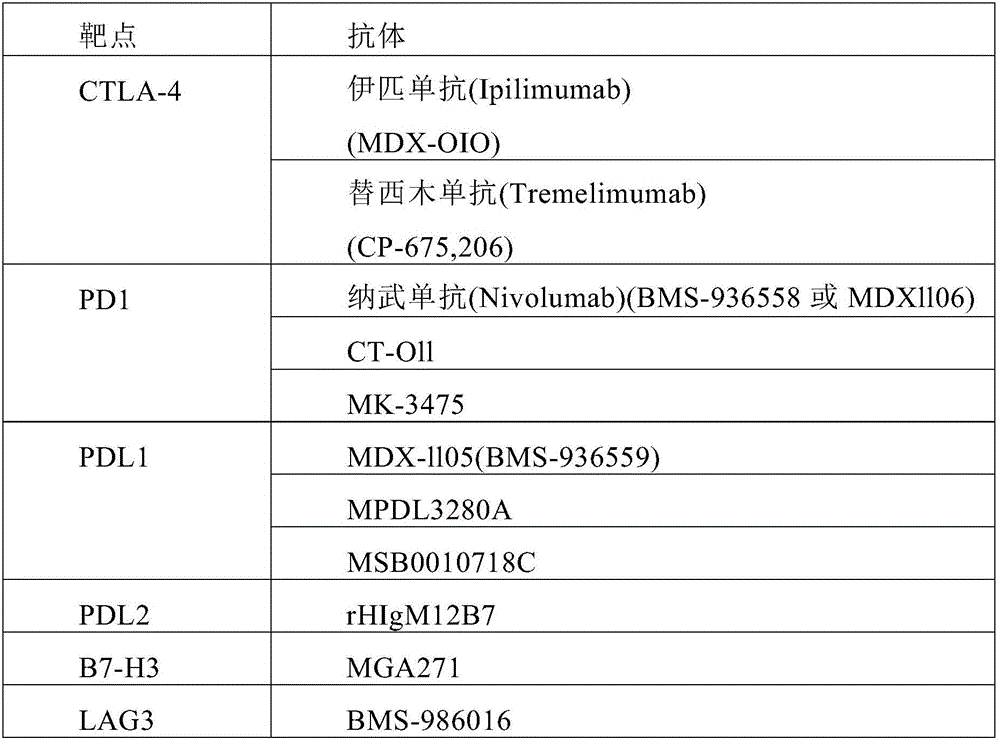
A method is disclosed for treating a cancer in a subject. The method comprises administering to the subject a composition comprising a therapeutically effective amount of a checkpoint inhibitor and a therapeutically effective amount of a tumor vaccine. In some embodiments, the tumor vaccine comprises radiated autologous tumor cells and a cell line engineered to express granulocyte- macrophage colony-stimulating factor (GM-CSF) and cluster of differentiation 40 (CD40) ligand. In some embodiments, the checkpoint inhibitor comprises an anti-programmed death-1 (anti-PD-1) antibody (e.g., BMS 936558), anti-programmed death ligand-1 (anti-PD-L1) antibody (e.g., cloneMIHI), anti-cytotoxic T lymphocyte antigen-4 (anti-CTLA-4) antibody (e.g., Ipilimumab, BMS), or any combination thereof.
Description
[0001] This application claims the benefit of US Provisional Application No. 62 / 171,829, filed June 5, 2015, the entire contents of which are incorporated herein by reference. Background technique [0002] It has been shown that cancer has multiple mechanisms to prevent the activation of the immune response and to evade it. Therefore, a single treatment, such as immunization with a vaccine, may not be sufficient for clinical efficacy. [0003] Summary of the invention [0004] Methods for treating cancer in a subject (subject) are disclosed. The method includes administering to the subject a composition comprising a therapeutically effective amount of a checkpoint inhibitor and a therapeutically effective amount of a tumor vaccine. In some embodiments, a tumor vaccine comprises irradiated (irradiated) autologous tumor cells and an engineered cell line expressing GM-CSF and a CD40 ligand. [0005] In some instances, the vaccine comprises a mixture of one, two or more human l...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K39/395A61K39/00
CPCA61K2039/505A61K2039/5152A61K2039/5156A61K2039/572A61K39/39541C07K2317/76C07K16/2818A61P31/00A61P11/00A61P35/00A61K39/001139A61K39/0011A61K2300/00A61K39/39558A61K45/05A61K2039/545
Inventor S·安东尼娅
Owner H LEE MOFFITT CANCER CENT & RES INST INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com