Cancer immunotherapy using autologous tumor cells combined with cells expressing a membrane cytokine
a technology of autologous tumor cells and membrane cytokine, which is applied in the field of cell immunology and cancer therapy, can solve the problems of high prognosis of cancer, and patients succumbing to recurrent or progressive disease, etc., and achieve the effect of suppressing systemic reactivity, eradicating or slowing the development of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
An Ovarian Cancer Cell Line Transduced to Express IL-4
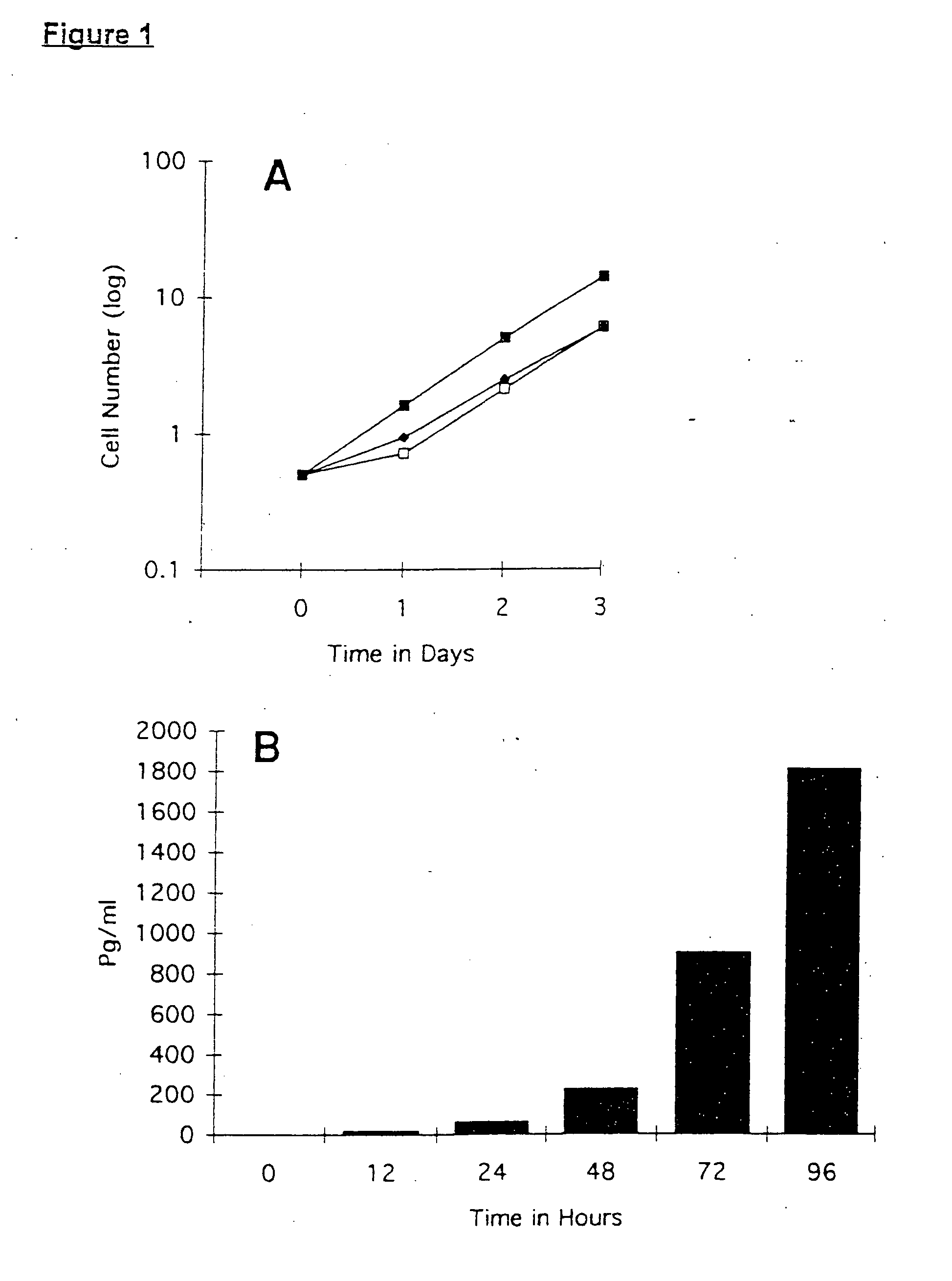
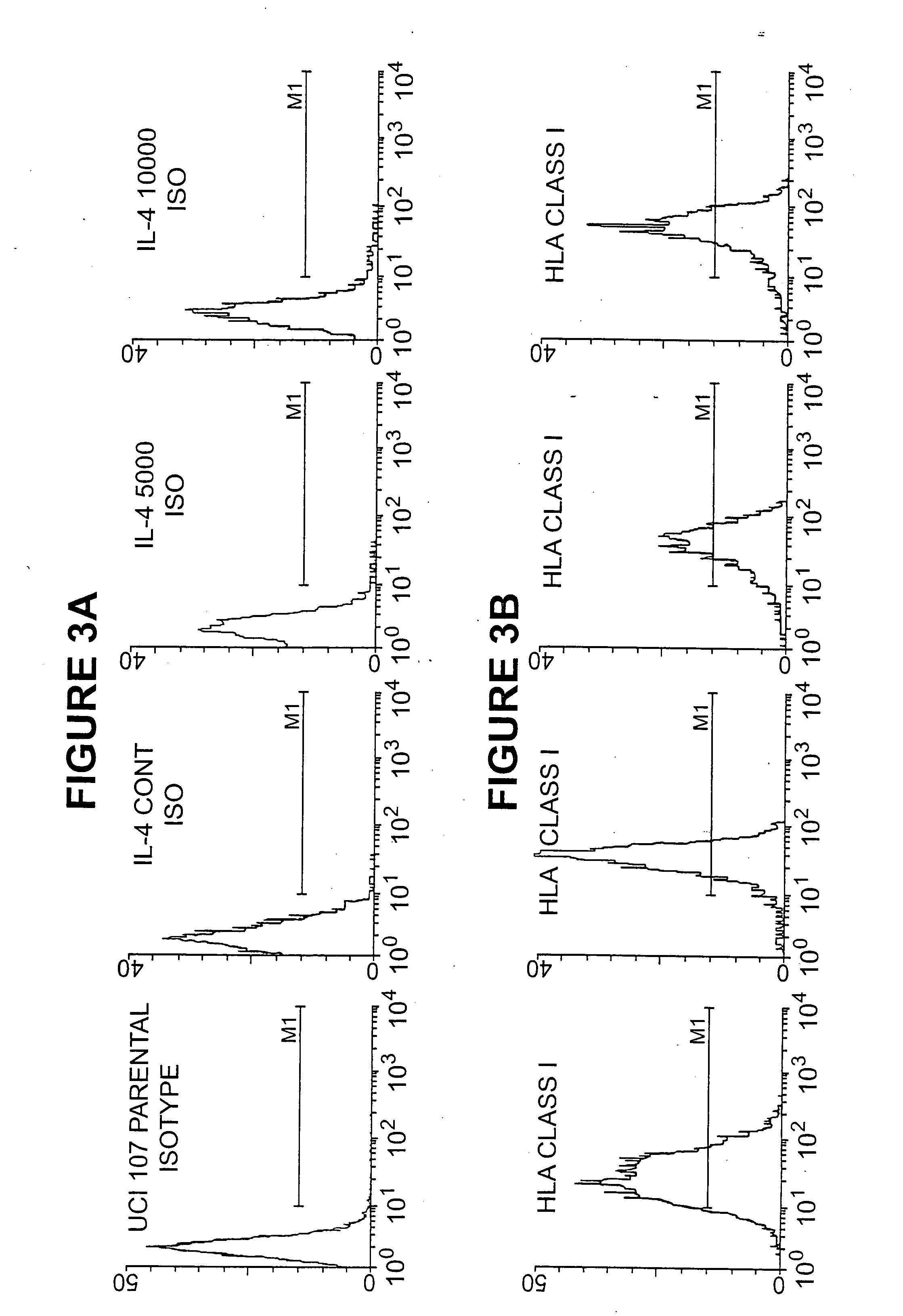
[0148] A human ovarian cancer cell line was genetically altered to secrete IL-4, using a retroviral vector comprising an IL-4 encoding construct. The cell line was stable, and capable of IL-4 biosynthesis even after an inactivating dose of radiation. The cell line expressed MHC Class I and Her-2 / neu antigens, but no MHC Class II antigens, ICAM-1, CA-125, or IL-4 receptors.
[0149] The human ovarian cell line UCI-107 was established from a previously untreated patient with a primary Stage III serous papillary adenocarcinoma of the ovary. The UCI-101 and UCI-107 cell lines have been previously characterized (Gamboa-Vujicic et al. Submitted, Gynecol. Oncol.) and were kindly provided by Dr. Alberto Manetta (University of California, Irvine Medical Center). Cells were maintained at 37° C., 5% CO2 in complete media (CM) containing RPMI 1640 (Gibco Life Technologies), 10 percent fetal bovine serum (FBS, Gemini Bioproducts, Calabassas, C...
example 2
An Ovarian Cancer Cell Line Transduced to Express GM-CSF
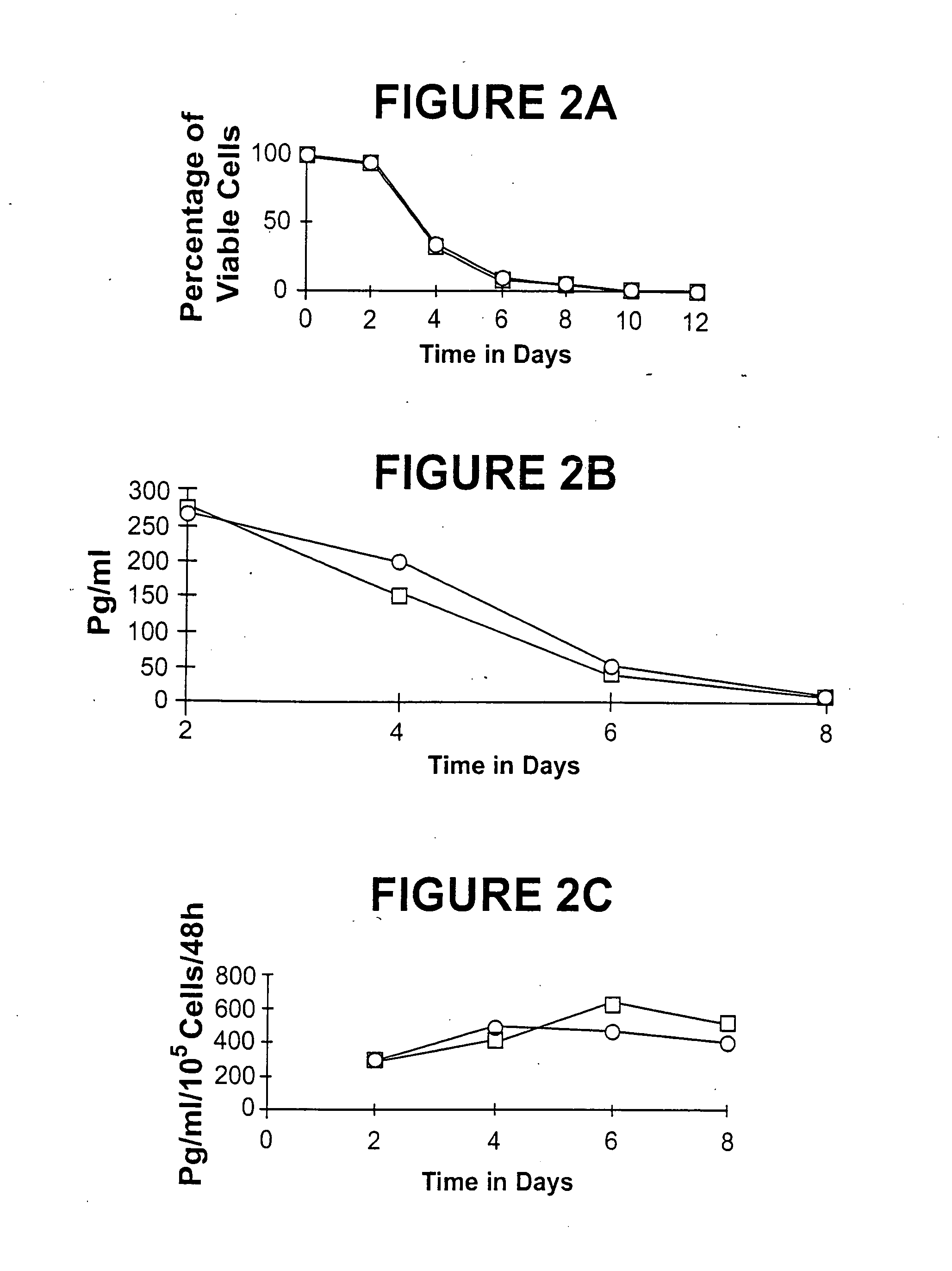
[0162] A human ovarian carcinoma cell line (UCI-107) was genetically engineered to secrete human cytokine granulocyte-macrophage colony stimulating factor (GM-CSF), similar to the method described in Example 1. One clone, termed UCI-107M GM-CSF-MPS, constitutively secretes high levels of GM-CSF (˜500 pg / ml / 105 cells 48 hours) UCI-107M GM-CSF-MPS cells express MHC Class I and Her2 / New surface antigens, but do not express detectable MHC Class II, ICAM-1 or the tumor-associated antigen CA-125. After a radiation dose of 10,000 rads, GM-CSF secretion continued until about Day 8.
[0163] The choice of transducing an ovarian carcinoma cell line with the GM-CSF gene has been made in light of the important role of GM-CSF in the maturation and function of specialized antigen-presenting cells. GM-CSF is one of the most potent stimulators of systemic anti-tumor immunity.
[0164] The pLXSN plasmid is described in Example 1. The human GM-CSF ...
example 3
An Ovarian Cancer Cell Line Transduced to Express IL-2
[0173] A human ovarian carcinoma cell line (UCI-107) was genetically engineered to secrete the cytokine Interleukin-2 (IL-2), by retroviral mediated gene transduction similar to the method outlined in Example 1. This line was transduced with the LXSN retroviral vector containing the human IL-2 gene and the neomycin resistance selection marker. One clone termed UCI-107A IL-2 AS, was shown to constitutively secrete high levels of IL-2 (i.e., 2,000 to 2,300 pg / ml / 105 cells / 48 hours) for over 35 passages and six months of study. Unlike parental and vector transduced cells (both of which were diploid), UCI-107A 1′-2 AS failed to express MHC Class I and Her2 / Neu surface antigens. In addition, UCI-107A IL-2 AS cells exhibited a distinct in vitro morphology, and were resistant to gamma irradiation.
[0174] The human IL-2 cDNA was obtained from ATCC in the Okayama and Berg pCD cloning vector and was excised using BamHI restriction enzyme....
PUM
| Property | Measurement | Unit |
|---|---|---|
| doubling time | aaaaa | aaaaa |
| doubling time | aaaaa | aaaaa |
| doubling time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com