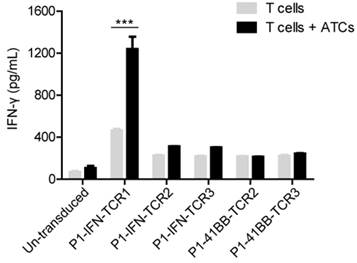
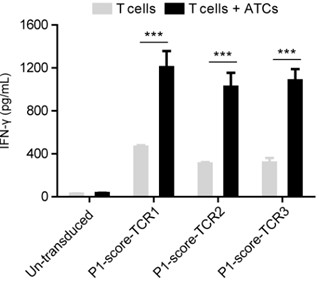
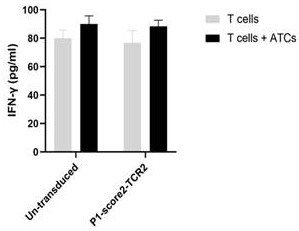
Method for screening tumor specific TCR
A TCR-T, tumor technology, applied in the field of tumor immunity, can solve the problems of large differences in expression levels, missing tumor-specific T cells, false negatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Sequencing of single-cell transcriptome and TCR group of TILs
[0032] Culture of TILs
[0033] After the tumor tissue of tumor patients was surgically removed, the tumor tissue was minced into small pieces of 1-2 mm, and each small piece of tumor tissue was placed in one well of a 24-well cell culture plate, and then T cell culture medium was added. T cell culture medium containing X-VIVO 15 serum-free medium (Lonza, USA) and IL2 (50U / ml; Peprotech, USA), IL-7 (10 ng / mL; Peprotech, USA), IL-15 (10 ng / ml; Peprotech, USA) / mL, Peprotech, USA), OKT3 antibody (50ng / ml; ACRO, USA) and anti-CD28 antibody (1ug / ml; T&L Biotechnology, China). The small pieces of tumor tissue were cultured in a cell incubator until final TILs were obtained.
[0034] Co-incubated with corresponding tumor cells
[0035] TILs and corresponding autologous tumor cells were co-incubated in X-VIVO15 serum-free medium for 12 hours, and then washed with PBS. According to 10Xgenomic requireme...
Embodiment 2
[0056] Example 2 Construction of TCR-T lentiviral vector
[0057] (1) In Example 1, a total of 21 TCRs were found, but since the partial TCRs of TCR1-3 obtained by different test groups were the same, the above 9 non-repetitive TCRs were synthesized in the present application, and each TCR core was used in each TCR core. The two ends of the nucleotide sequence were added with XbaI and SalI restriction sites respectively, and cloned into the pUC57 vector;
[0058] (2) The pUC57 vector containing the target gene was digested with XbaI and SalI double enzymes, and the target gene fragment was recovered by cutting gel;
[0059] (3) The original vector pCDH-EF1-Luc2-T2A-tdTomato was digested with XbaI and SalI double enzymes, and the vector fragment of about 6.5kb was recovered by cutting gel;
[0060] (4) Use DNA ligase to connect the recovered target gene and vector fragment to obtain a recombinant lentiviral vector carrying each TCR.
Embodiment 3
[0061] Example 3 Preparation of TCR lentivirus
[0062] The 9 recombinant lentiviral vectors of Example 2 were transfected into 293ft cells by transfection reagent (PEI) to produce lentivirus. The specific method includes: packaging plasmid mixture (pMDL:VSV-G:REV=5:3:2, mass ratio) and TCR1 lentiviral vector in 500 μL serum-free medium Opti-MEM at a mass ratio of 1:1, vortex Swirl until well mixed. Add 32 g PEI to 500 μL of serum-free medium Opti-MEM and vortex to mix well. Then, 500ul of the plasmid mixture was mixed with 500ul of PEI, and it was added to 293ft cells with a confluence of about 90%. After 48 hours, the virus supernatant was collected, and after ultracentrifugation, the virus was concentrated 100 times to obtain the concentrated virus.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com