A fusion lyase for lysing Escherichia coli
A technology of Escherichia coli and colicin, which is applied in the field of fusion lysing enzymes, can solve the problem that Escherichia coli cannot be lysed from the outside
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Construction and purification of fusion lyase
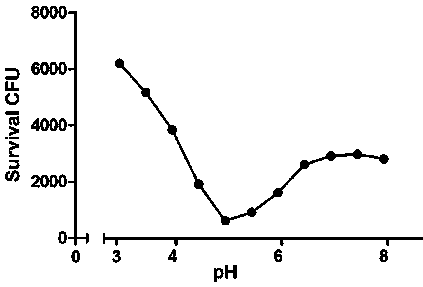
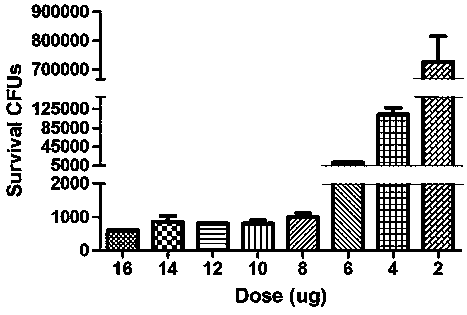
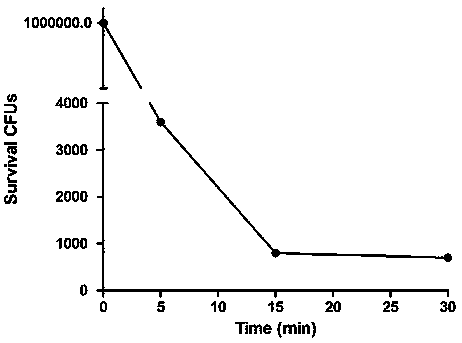
[0023] The gene of the colicin transit fragment (SEQ NO.1) was cloned by PCR, and the recombinant plasmid pUC-colicin was constructed on the serialized T-vector, and the gene of the phage lyase (SEQ NO.1) was cloned by PCR. The gene of SEQ NO.2), the serial pUC-colicin, and the downstream of the colicin transit fragment were constructed into recombinant plasmid pUC-lysis. The fused colibactin transit fragment and the gene of phage lyase (SEQ NO.3) were excised with endonuclease and recovered. The recovered product was connected to the prokaryotic expression vector pET-28a to construct the recombinant prokaryotic expression vector pET-lysis. Transform pET-lysis into BL21(DE3) condon Plus RIPL. Bacteria were grown to logarithmic growth phase (OD=0.45) at 37°C. After being induced by adding IPTG, the bacterial culture containing the colicin-lysis gene carrier would suddenly become clear at a certain time after adding IPT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com