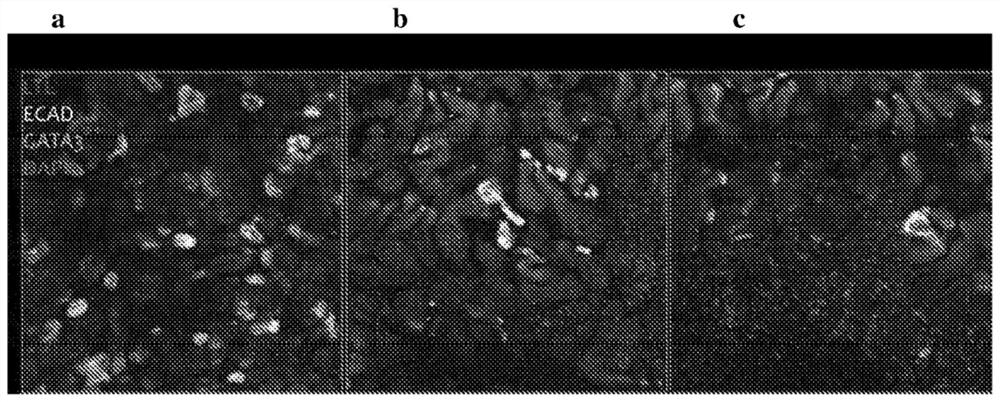
Differentiation of pluripotent stem cells forming nephronoids
A technology of pluripotent stem cells, fibroblasts, applied in the field of in vitro generation of nephronoids, which can solve the problems of lack of cell types, obstacles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212] Materials and methods
[0213] Non-limiting examples of sources of reagents mentioned in these methods are provided in Table 1.
[0214] The culture medium is as follows:
[0215] • Gelatin solution: 0.1% gelatin in PBS. The solution was then autoclaved.
[0216] · FDMEM: 89% DMEM High Glucose, 10% Fetal Bovine Serum, 1% GlutaMAX Supplement, 0.5% Penicillin / Streptomycin.
[0217] • KSR medium: 77.8% DMEM / F-12, 20% Knockout Serum Replacement, 1% NEAA, 1% GlutaMAX, 0.5% Penicillin / Streptomycin, 0.2% 2-mercaptoethanol (55 mM). The medium was then filtered with Stericup-GP.
[0218] • MEF-conditioned KSR medium: Add 40 mL of KSR medium to 10 million mouse embryonic fibroblasts in a T175 flask for a day. The medium was collected the next day, and 40 mL of KSR medium was added. After repeating 6 times, the collected and pooled media were filtered through Stericup-GP.
[0219]• APEL: Supplement one vial of STEMdiff APEL (100 mL) with 0.5 mL antibiotic-antimycotic.
...
Embodiment 2
[0316] Cell Culture and Differentiation
[0317] All presented experiments used the previously described wild-type human iPSC line CRL1502 (clone #C32), which uses episomal reprogramming 28 produce. Undifferentiated human iPSCs were maintained on mouse embryonic fibroblasts (MEFs) (Millipore) as a feeder layer using human ES cell (hES) medium as described previously 1 . Characterize cells and detect mycoplasma infection 28 . Human iPSCs were plated on Matrigel-coated (Millipore) dishes and cultured in MEF-conditioned hES medium (MEF-CM) until reaching 60-100% confluency. Then, the cells were grown again at 5000 cells / cm 2 Plate on Matreigel-coated dishes and culture in MEF-CM. The next day, cells reached 40–50% confluency, and cells were treated with 8 μM CHIR99021 for 2–5 days in APEL basal medium (STEMCELL Technologies) supplemented with antibiotic-antimycotics (Life Technologies) and then treated with FGF9 (200 ng mL -1) and heparin (1μg mL -1 ) for another 5-2 days...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com