Methods for generating hepatocytes and cholangiocytes from pluripotent stem cells
A technology of pluripotent stem cells and cholangiocytes, applied in the field of generating hepatocytes and cholangiocytes from pluripotent stem cells, which can solve problems such as no description
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0395] Endoderm induction in EBs
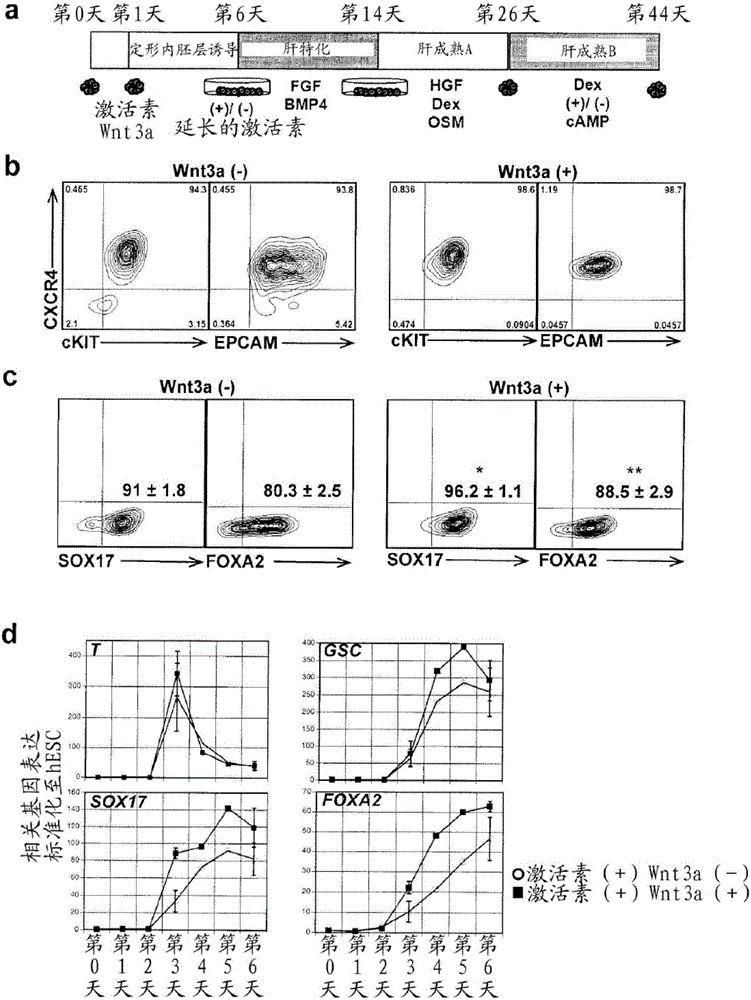
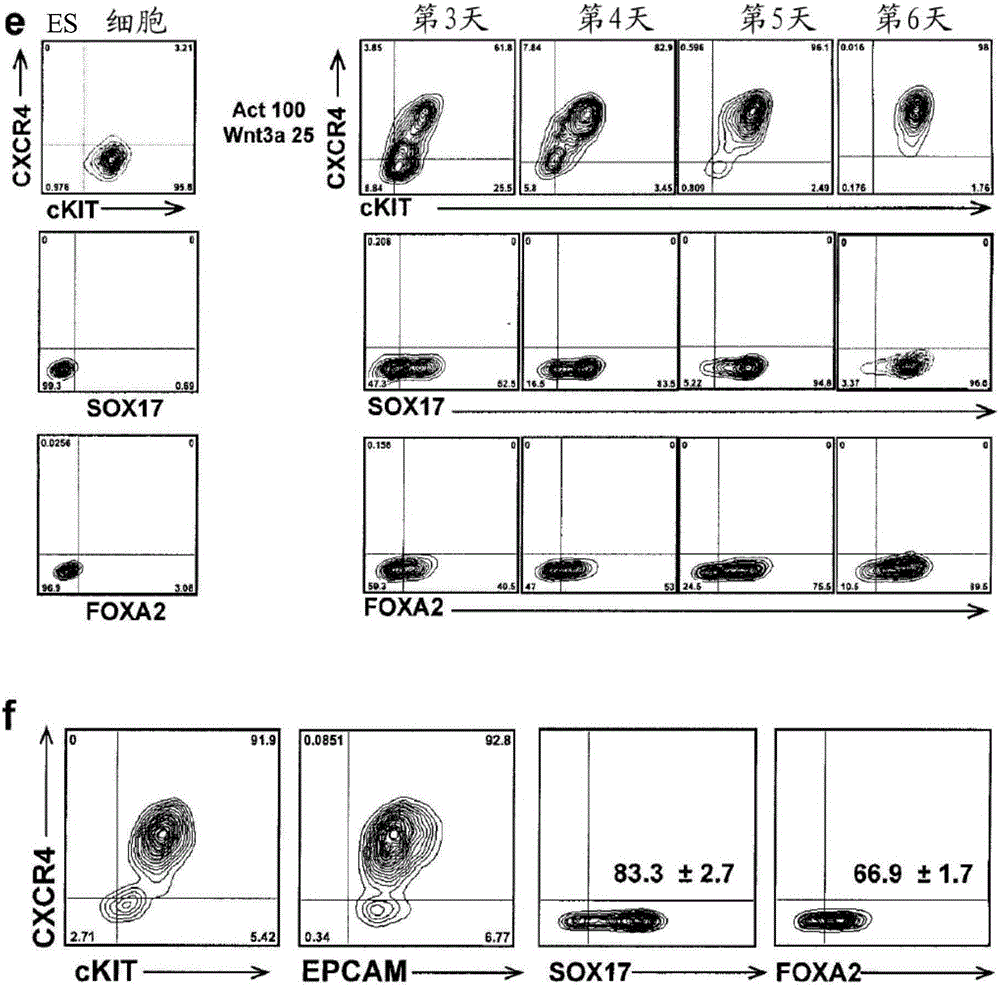
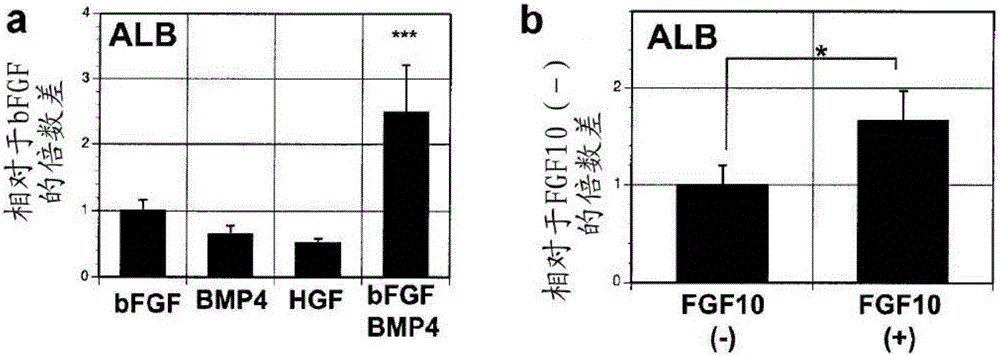
[0396] figure 1 In a, the strategy for generating hepatocytes from hESCs using embryoid bodies (EBs) is shown. Similar to protocols using monolayer cultures 16 , which involves a specification step that recapitulates key stages of liver development in early embryos. as previously described 18 , EBs formed from small aggregates cultured for 24 hours in low levels of BMP4. EBs were subsequently exposed to high concentrations of activin A (hereinafter referred to as activin) for five days to induce definitive endoderm, defining populations by the expression of surface markers CXCR4, CKIT, and EPCAM, and transcription factors SOX17 and FOXA2. Such as figure 1 As shown in b, more than 90% of the induced EB population co-expressed CXCR4 and CKIT or CXCR4 and EPCAM after five days of activin induction (6 days of co-culture). Intracellular flow cytometric analysis indicated that more than 90% of the population expressed SOX17, and more than 80%...
example 2
[0448] CHIR99021 is a selective inhibitor of GSK3 that has been reported to mimic the canonical Wnt signaling pathway. CHIR99021 was tested as a replacement for wnt3a (eg, added in combination with activin) in induction of embryoid bodies and for monolayer induction of definitive endoderm from hPSCs. as seen Figure 8 a) and Figure 8 b) Replacement of Wnt3a with CHIR99021 induced a proportion of CKIT+CXCR4+ and CXCR4+EPCAM+ cells greater than 95% of the population and equivalent to that seen in activin / wnt3a induction.
[0449] Figure 8 (a) and Figure 8 (b) demonstrates that CHIR99021 can induce definitive endoderm cells. Figure 8 (a) is a flow cytometric analysis showing the proportion of CXCR4+, CKIT+ and EPCAM+ cells in activin / CHIR99021 at day 6 of embryoid body induction with activin / wnt3a. Figure 8 (b) is a flow cytometric analysis showing the proportion of CXCR4+, CKIT+ and EPCAM+ cells in activin / CHIR99021 at day 6. Figure 8 (c) and Figure 8 (d) shows day...
example 3
[0455] Ectopic liver tissue in NSG mice
[0456] Transplanted hESC-derived hepatocytes engraft and generate cells expressing hepatocyte differentiation markers.
[0457] Figure 8 (e), Figure 8 (f) and Figure 8 (g) demonstrates the engraftment of ES-derived hepatocytes prepared using the method described in Example 1, except that the GSK3 inhibitor CHIR99021 was used to make the transplanted cells described in Example 2.
[0458] Figure 8 (e), Figure 8 (f) and Figure (g), Figure 8 (h) Ectopic liver tissue in NSG mice. Figure 8 (e) is an exemplary photomicrograph of H&E staining of the mesenteric region showing clusters of hESC-derived hepatocytes (arrows) 2 months after transplantation. The magnification is 5x. Intestine (arrow), implanted cells (head of arrow). Figure 8 (f) show from Figure 8 High magnification (10×) photomicrograph of the H&E stained section of (e).
[0459] Figure 8 (g), Figure 8 (h) Immunohistochemical staining showing the presence o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com