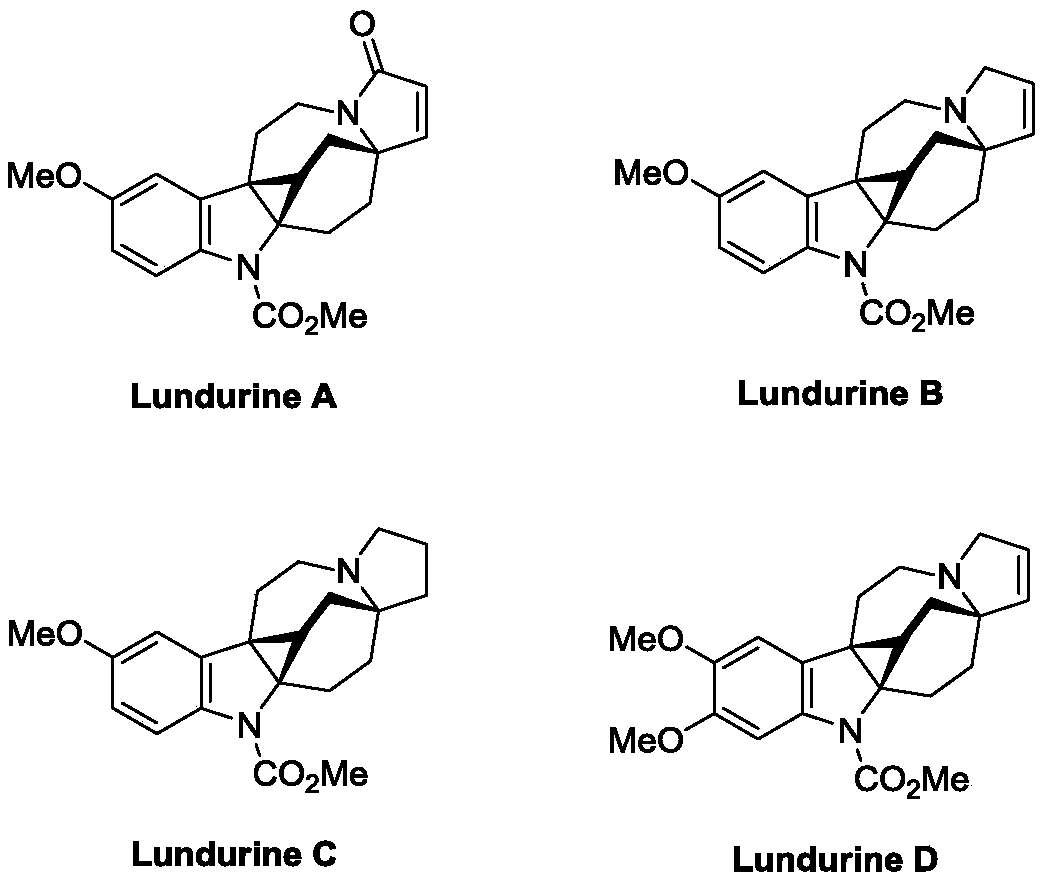
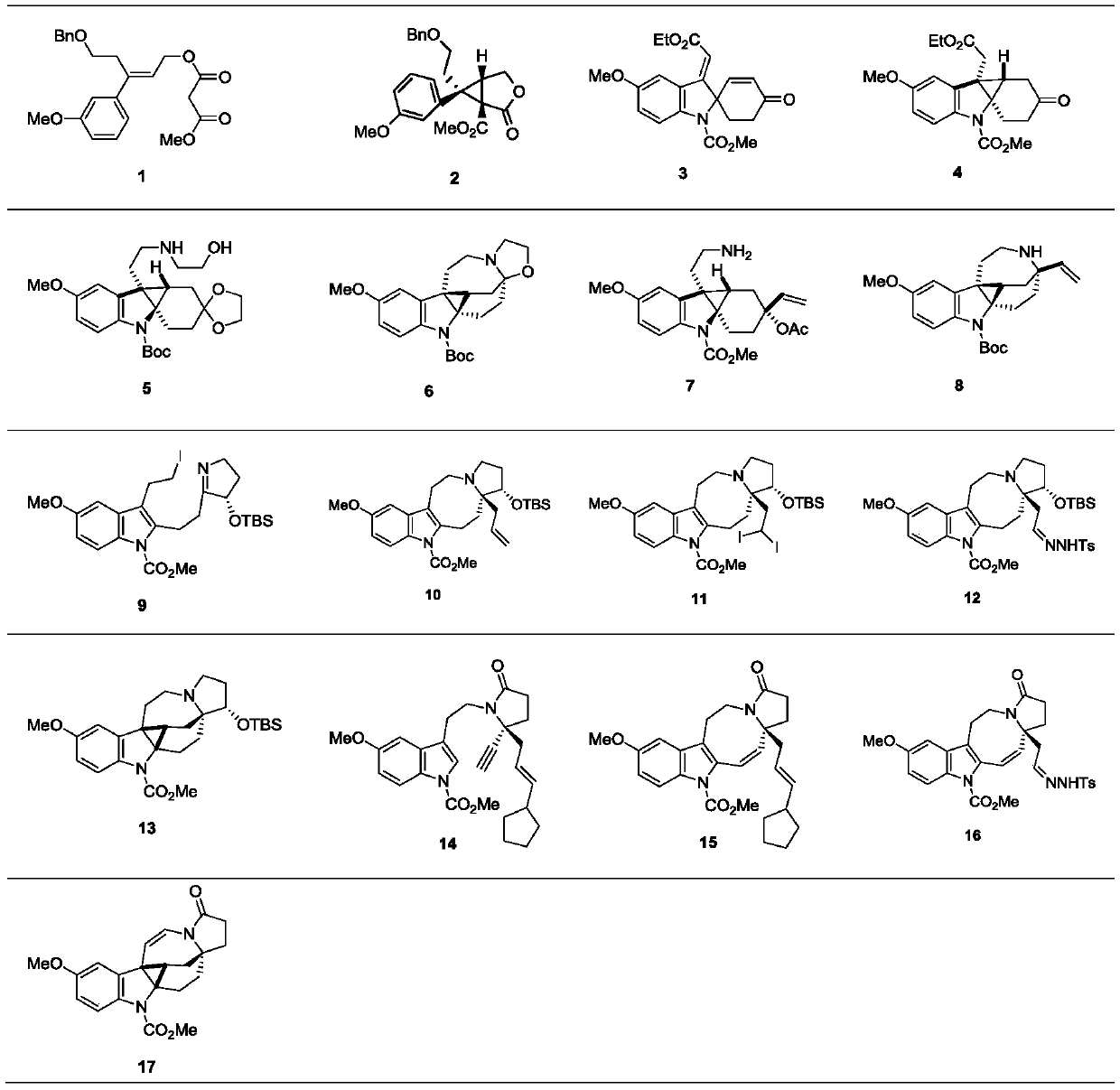
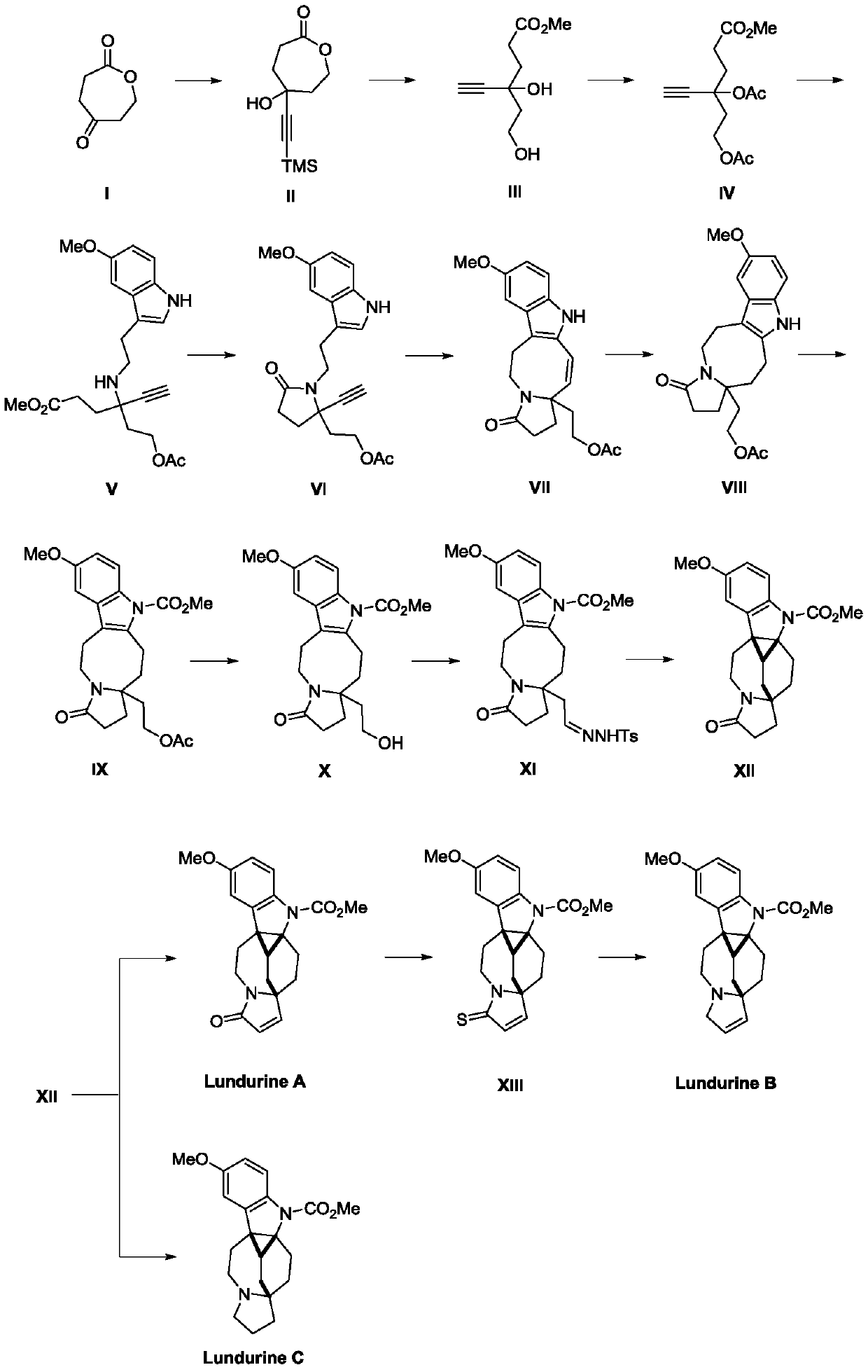
Preparation method of natural alkaloid lundurine A-C
An alkaloid, natural technology, applied in the direction of organic chemistry, etc., can solve the problems of long reaction steps, harsh reaction conditions, expensive reaction conditions of reaction reagents, etc., and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] Preparation of compound TMS-based lactone II (intermediate II):
[0011] Add a stirring bar to a 25mL round bottom flask, replace nitrogen, add trimethylsilylacetylene (4.4mL, 11mmol), anhydrous and oxygen-free THF (15mL), cool at -78°C for 30min, add n-butyllithium (11mmol) dropwise for 30min , -78 ℃ should be 60 minutes. A 100mL round-bottomed flask filled with I (1.28g, 10mmol) was replaced with nitrogen, anhydrous tetrahydrofuran (55mL) was added, lithium reagent was added dropwise to the reaction flask at -78°C for 60 minutes, and reacted at -78°C for 2 hours (thin layer analysis detection). The reaction was quenched with sodium acetate / acetic acid buffer solution (pH=6.5), and extracted with ethyl acetate. The organic phase was washed with water and saturated sodium chloride, and dried over anhydrous sodium sulfate. After filtration, the mother liquor was concentrated under reduced pressure; the concentrate was purified by silica gel column chromatography (petr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com