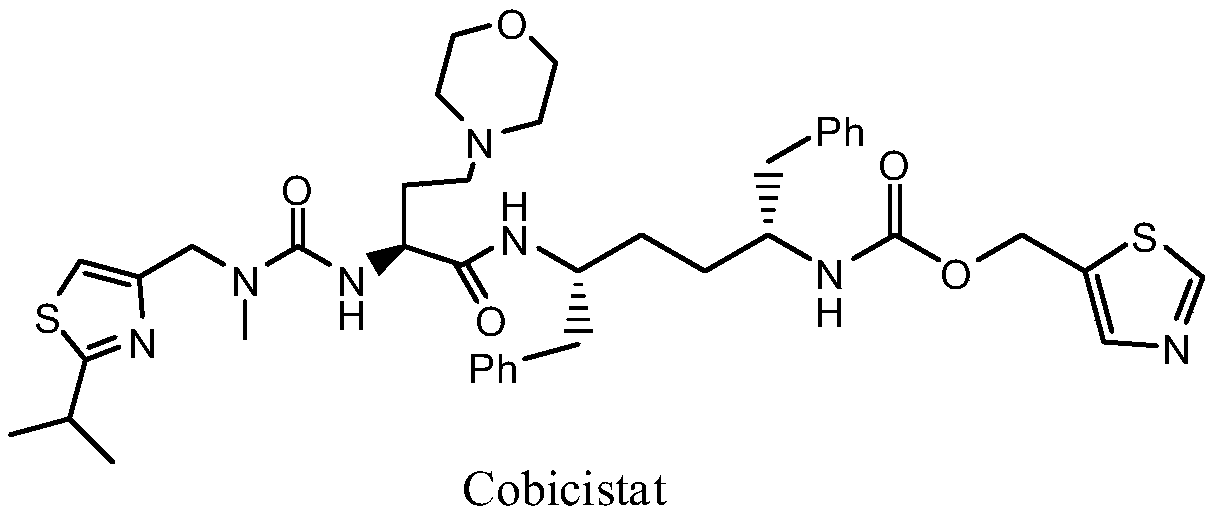
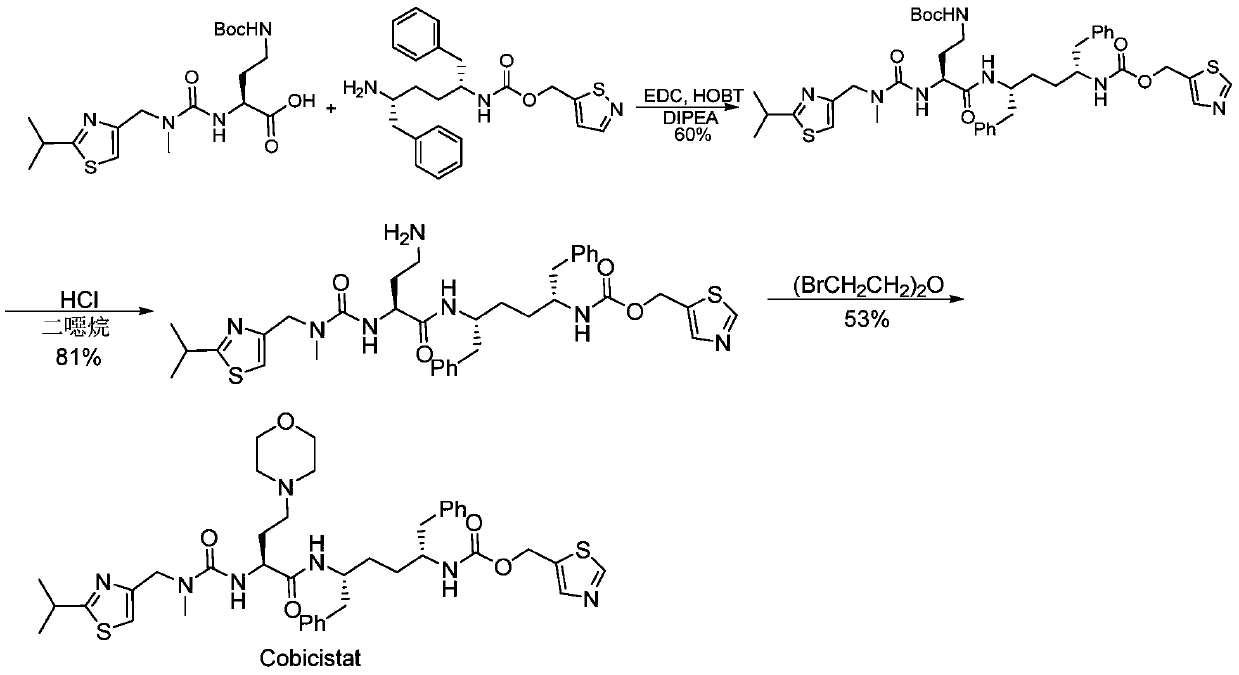
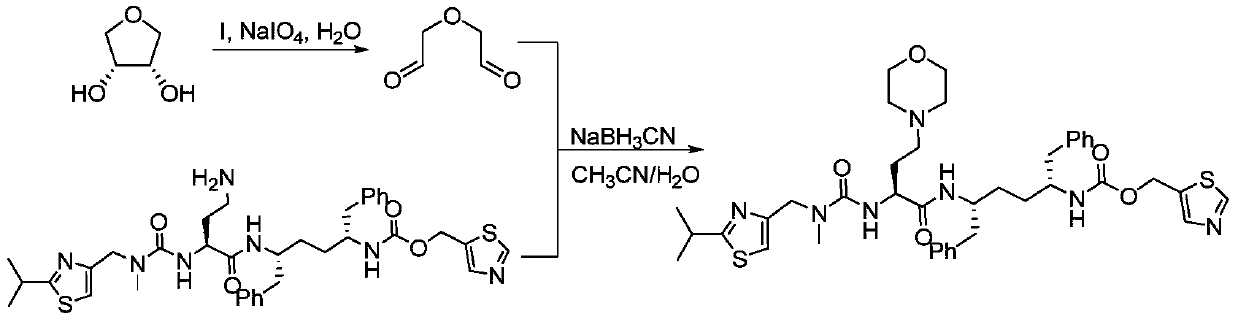
A kind of synthetic method of cobicistat
A synthesis method and compound technology, which can be applied in the direction of organic chemistry, etc., can solve the problems such as the long route of comparablystatin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of Compound III:
[0031] Compound II (see CN104876888A for the synthesis method) (100g, 0.29mol, 1 equiv) was dissolved in dichloromethane (90ml), cooled to -20~-18°C, and compound I (see CN104876888A for the synthesis method) (0.35mol, 1.2 equiv), control the temperature -20~-18℃ and react for 1h. HOBT (0.41 mol, 1.4 equiv) was added and stirred for 1 h. A pre-cooled -20°C EDC·HCl (0.58mol, 2equiv) dichloromethane solution (130ml) was added, the temperature was controlled below -20°C, and the reaction was completed in 24h. The reaction was warmed to 3°C and quenched with 10% citric acid solution (100 ml). Separation, the organic phase was washed with 15% sodium bicarbonate solution (145ml) and water (45ml) respectively, the organic phase was distilled under reduced pressure, and then co-evaporated with absolute ethanol to obtain compound III (0.28mol) with a yield of 96%.
[0032] 1 H NMR (500MHz, Chloroform): δ 1.39-1.42 (m, 8H), 1.54-1.56 (m, 2H), 1.57...
Embodiment 2
[0038] Synthesis of compound III:
[0039] Dissolve compound II (100g, 0.29mol, 1equiv) in dichloromethane (90ml), cool down to -20~-18°C, add compound I (0.35mol, 1.2equiv), control the temperature -20~-18°C for reaction 1h. Add DMAP (0.41 mol, 1.4 equiv) and stir for 1 h. A pre-cooled -20°C EDC·HCl (0.58mol, 2equiv) dichloromethane solution (130ml) was added, the temperature was controlled below -20°C, and the reaction was completed within 24h. The temperature of the reaction system was raised to 3° C., and the reaction was quenched with 10% citric acid solution (95 ml). Liquid separation, the organic phase was washed with 15% sodium bicarbonate solution (145ml) and water (45ml) respectively, the organic phase was distilled under reduced pressure, and then co-evaporated with absolute ethanol to obtain the product (0.28mol) with a yield of 96%.
[0040] Synthesis of Cobicistat:
[0041] Under nitrogen protection, compound III (100g, 0.16mol, 2equiv) was dissolved in dichl...
Embodiment 3
[0044] Synthesis of compound III:
[0045] Compound II (100g, 0.29mol, 1equiv) was dissolved in toluene (90ml), cooled to -20~-18°C, compound I (0.35mol, 1.3equiv) was added, and the temperature was controlled at -20~-18°C for 1h. Add N,N'-carbonyldiimidazole (0.41mol, 1.4equiv) toluene solution, control the temperature below -20°C, and complete the reaction within 24h. The temperature of the reaction system was raised to 3° C., and the reaction was quenched with 10% citric acid solution (95 ml). The liquid was separated, the organic phase was washed with 15% sodium bicarbonate solution (145ml) and water (45ml) respectively, the organic phase was distilled under reduced pressure, and then co-evaporated with absolute ethanol to obtain the product (0.27mol) with a yield of 93%.
[0046] Synthesis of Cobicistat:
[0047] Under the protection of nitrogen, compound III (100g, 0.16mol, 2equiv) was dissolved in toluene (300ml), compound IV (0.104mol, 1.3equiv) was added, after stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com