A kind of pentapeptide modified rhodamine b compound and its preparation method and application
A compound and modified technology, applied in the field of medicine, can solve the problems of poor tumor imaging effect, less research, fast metabolism, etc., and achieve the effects of improving imaging contrast and clarity, good water solubility, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
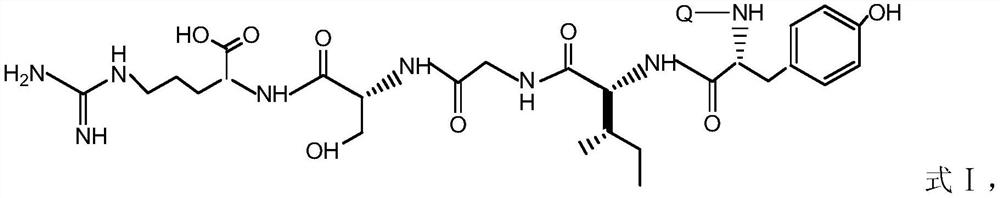
[0025] Preparation of pentapeptide modified rhodamine B compound, the steps are as follows:
[0026] 1) Pentapeptide (tyrosyl-isoleucyl-glycyl-seryl-arginine, YIGSR) was prepared by solid-phase peptide synthesis;
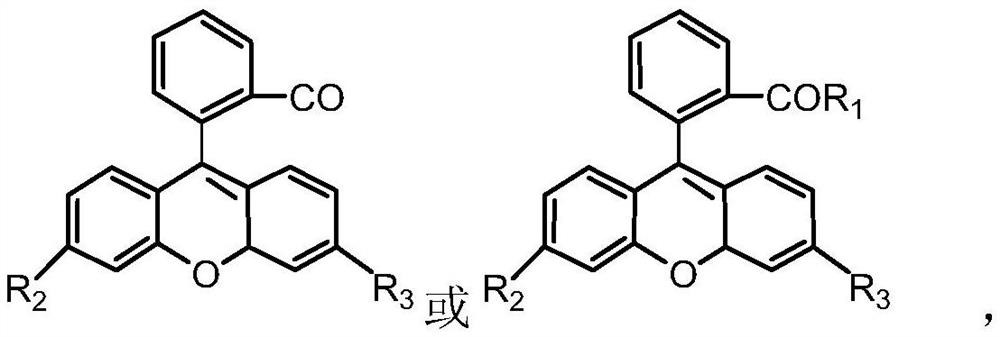
[0027] 2) Add 0.5g pentapeptide (tyrosyl-isoleucyl-glycyl-seryl-arginine, YIGSR, 0.84mmol) into the container, add 9-(2-carboxyphenyl)-3 , 6-bis(diethylamino)xanthenium chloride (rhodamine B, 0.4g, 0.84mmol) and diisopropylethylamine (0.11g, 0.84mmol) in DMF (10mL), stirred for 1.5 hours , filtered, and the filtrate and washings were collected, concentrated by rotary evaporation, and added dropwise to cold ether to obtain a red precipitate, which was filtered and washed with suction to obtain 0.51 g of pentapeptide modified rhodamine B compound red solid powder, with a yield of 60%.
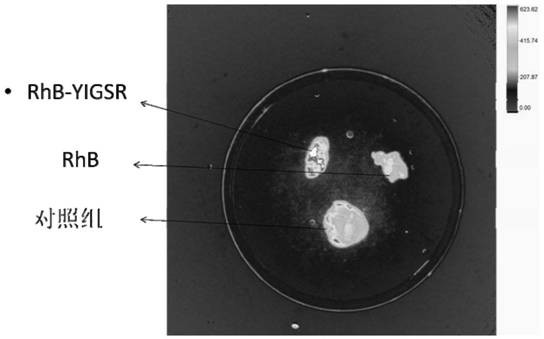
[0028] Test the effect of the pentapeptide modified rhodamine B compound prepared in this embodiment as a tracer for fluorescence imaging, and use the same dose of rhodamine B as a co...
Embodiment 2
[0031] Preparation of pentapeptide modified rhodamine B compound, the steps are as follows:
[0032] 1) Pentapeptide (tyrosyl-isoleucyl-glycyl-seryl-arginine, YIGSR) was prepared by solid-phase peptide synthesis;
[0033] 2) Add 1g of pentapeptide (tyrosyl-isoleucyl-glycyl-seryl-arginine, YIGSR, 1.68mmol) into the container, add 9-(2-carboxyphenyl)-3, 6-bis(amino)xanthenium chloride (0.62g, 1.68mmol) and diisopropylethylamine (0.22g, 1.68mmol) in DMF (20mL), stirred for 2 hours, filtered, and the filtrate was collected and washed solution, concentrated by rotary evaporation, and added dropwise into cold ether to obtain a red precipitate, which was filtered and washed to obtain 1.11 g of pentapeptide modified rhodamine B compound red solid powder, with a yield of 70%.
Embodiment 3
[0035] Preparation of pentapeptide modified rhodamine B compound, the steps are as follows:
[0036] 1) Pentapeptide (tyrosyl-isoleucyl-glycyl-seryl-arginine, YIGSR) was prepared by solid-phase peptide synthesis;
[0037]2) 5g pentapeptide (tyrosyl-isoleucyl-glycyl-seryl-arginine, YIGSR, 8.4mmol), 9-(2-carboxyphenyl)-3,6-bis( Hydroxy)xanthenium compound (0.4g, 8.4mmol), dicyclohexylcarbodiimide (DCC, 1.73g, 8.4mmol) and triethylamine (0.85g, 8.4mmol) were dissolved in dimethyl sulfoxide solution ( 100 mL), stirred and reacted in an ice-water bath for 4 hours, precipitated with a mixed solvent of ethanol / ether (volume ratio 1 / 5), and collected the solid by filtration. Then the solid was dissolved with DMF, and then reprecipitated with ethanol / ether mixed solvent (volume ratio 1 / 5), the solid was collected by filtration, and vacuum-dried for 24 hours to obtain 6.16g pentapeptide modified Rhodamine B compound red solid powder, Yield 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com