Anti-human vascular endothelial growth factor monoclonal antibody drug preparation, and applications thereof
A monoclonal antibody, vascular endothelium technology, applied in the direction of antibodies, anti-inflammatory agents, anti-tumor drugs, etc., can solve problems affecting drug efficacy, toxic side effects, and unpublished pharmaceutical preparation components.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
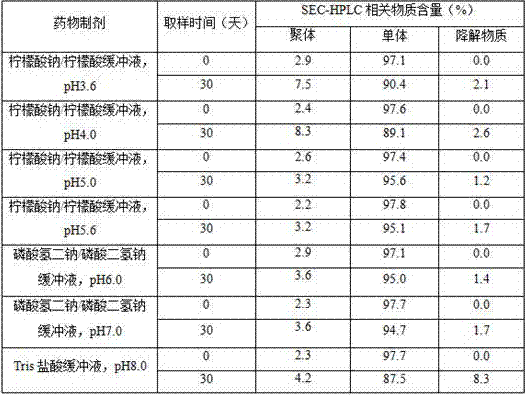
[0049] Example 1 Effects of different pH and buffer systems on the stability of 5 mg / ml (low concentration) anti-vascular endothelial growth factor monoclonal antibody preparations
[0050]Anti-VEGF single antigen solution was desalted into purified water. Add the prepared buffer mother liquor by final concentration 50 mmol / L (wherein pH 3.6, 4.0, 5.0 and 5.6 select sodium citrate / citric acid buffer, pH 6.0 and 7.0 select disodium hydrogen phosphate / sodium dihydrogen phosphate buffer for use , pH 8.0 using Tris hydrochloric acid buffer), and adjust the final concentration of anti-vascular endothelial growth factor monoclonal antibody to 5 mg / ml, sterile filter. The samples of each group were divided into 2 ml vials, sealed and placed in a constant temperature incubator at 37 °C. The samples were taken on the 30th day, and the purity was detected by reducing SDS-PAGE and SEC-HPLC.
[0051] Table 1 SEC-HPLC purity of pharmaceutical preparations under different pH and buffer sys...
Example Embodiment
[0054] Example 2 Screening effects of different pH and buffer systems on the stability of 200 mg / ml (high concentration) anti-vascular endothelial growth factor monoclonal antibody preparations.
[0055] In order to investigate the influence of pH and buffer system on preparation stability, prepare different pH buffer solution mother liquors respectively (wherein pH 5.0, 5.5 and 6.0 select histidine / histidine hydrochloride buffer, pH 6.0, 6.5 and 7.0 select phosphoric acid for use. disodium hydrogen phosphate / sodium dihydrogen phosphate buffer). The anti-vascular endothelial growth factor single-antigen solution was concentrated by ultrafiltration and then replaced with pure water, and each buffer stock solution was added to the final concentration of 30 mmol / L. At the same time, adjust the anti-vascular endothelial growth factor monoclonal antibody to a final concentration of 200 mg / ml, and sterile filter. The pharmaceutical preparations of each group were divided into 2 ml ...
Example Embodiment
[0059] Example 3 Selection of protective agent
[0060] Long-term storage will lead to increased aggregation of anti-vascular endothelial growth factor drug preparations, mainly because the slow change of protein conformation leads to the exposure of certain hydrophobic regions, so that protein molecules aggregate with each other to form aggregates. Trehalose, mannitol, sucrose, arginine and other protective agents can interact with protein molecules to improve protein stability and inhibit the formation of aggregates.
[0061] 3.1 The effect of protective agents on protein thermal stability
[0062] In this example, the effects of methionine, lysine, arginine, glycine, sucrose, sorbitol, mannitol, maltose, trehalose, sucrose, arginine and other single components on the thermal stability of proteins were investigated; blank The control group was the disodium hydrogen phosphate / sodium dihydrogen phosphate buffer group, and no protective agent was added. The anti-vascular endo...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap