Selective progesterone receptor modulator (SPRM) regimen
A variant, solvate technology, applied in the field of treatment of heavy menstrual bleeding, can solve problems such as endometrial thickening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Embodiment 1: the synthetic route of compound 1
[0125] (11β,17β)-17-Hydroxy-11-[4-(methylsulfonyl)phenyl]-17-(pentafluoroethyl)estr-4,9-dien-3-one
[0126]
[0127] 5 g of the above compound were dissolved in a mixture of 140 ml THF and 140 ml methanol. At 0°C, slowly drop 20g Solution in 94ml of water. It was then stirred for a further 3.5 hours at 0°C. A mixture of water and dichloromethane was then added to the reaction mixture. The phases are separated and the aqueous phase is extracted several times with dichloromethane. The combined organic phases were washed with saturated aqueous sodium chloride, dried over sodium sulfate and concentrated in vacuo. The crude product was purified by silica gel chromatography. This gave 3.8 g of the title compound.
[0128] 1H-NMR (300MHz, CDCl3): δ=7.86d(2H); 7.40d(2H); 5.81sbr(1H); 4.50dbr(1H); 3.07s(3H); 0.51s(3H).
Embodiment 2
[0129] Example 2: Efficacy and Safety of Compound 1 in Patients Diagnosed with Uterine Fibroids:
[0130] Research proposal:
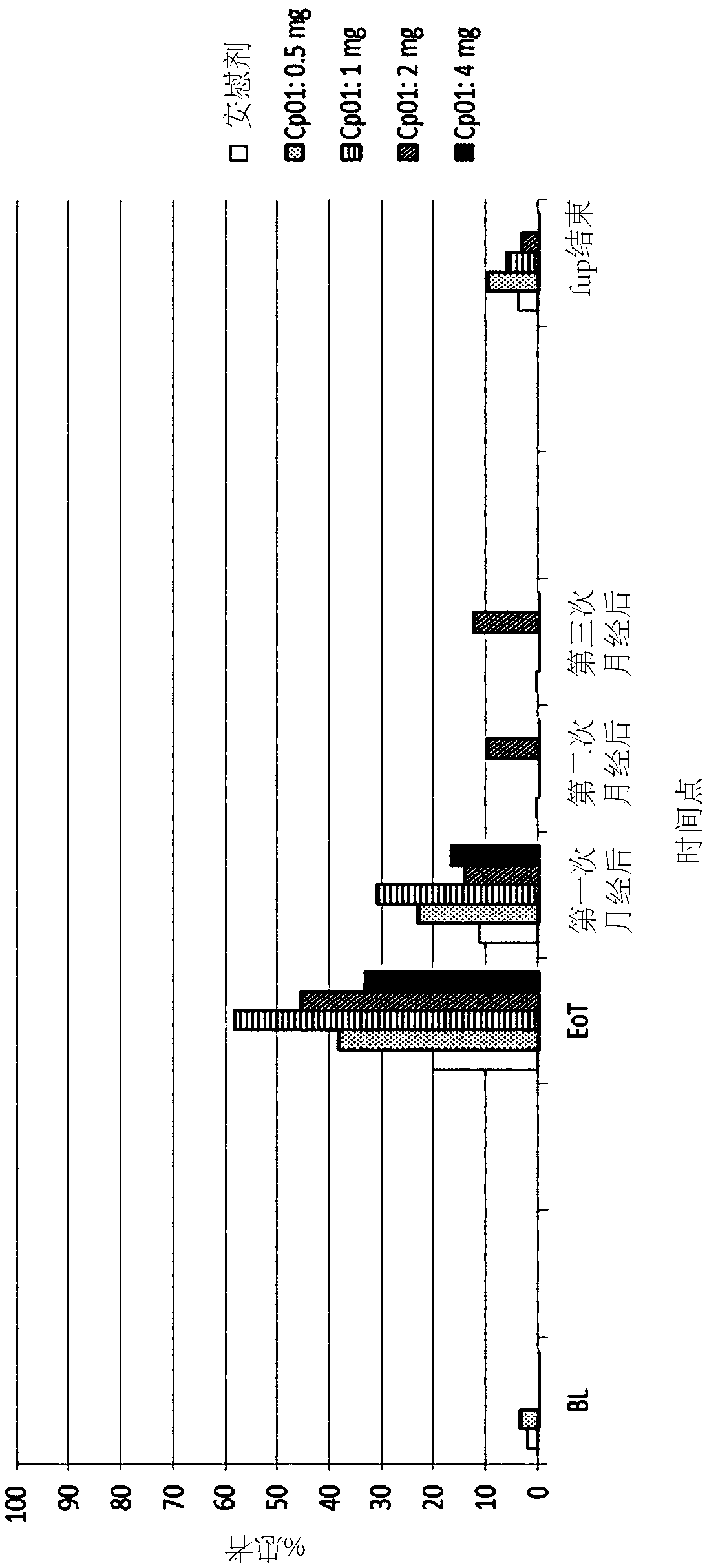
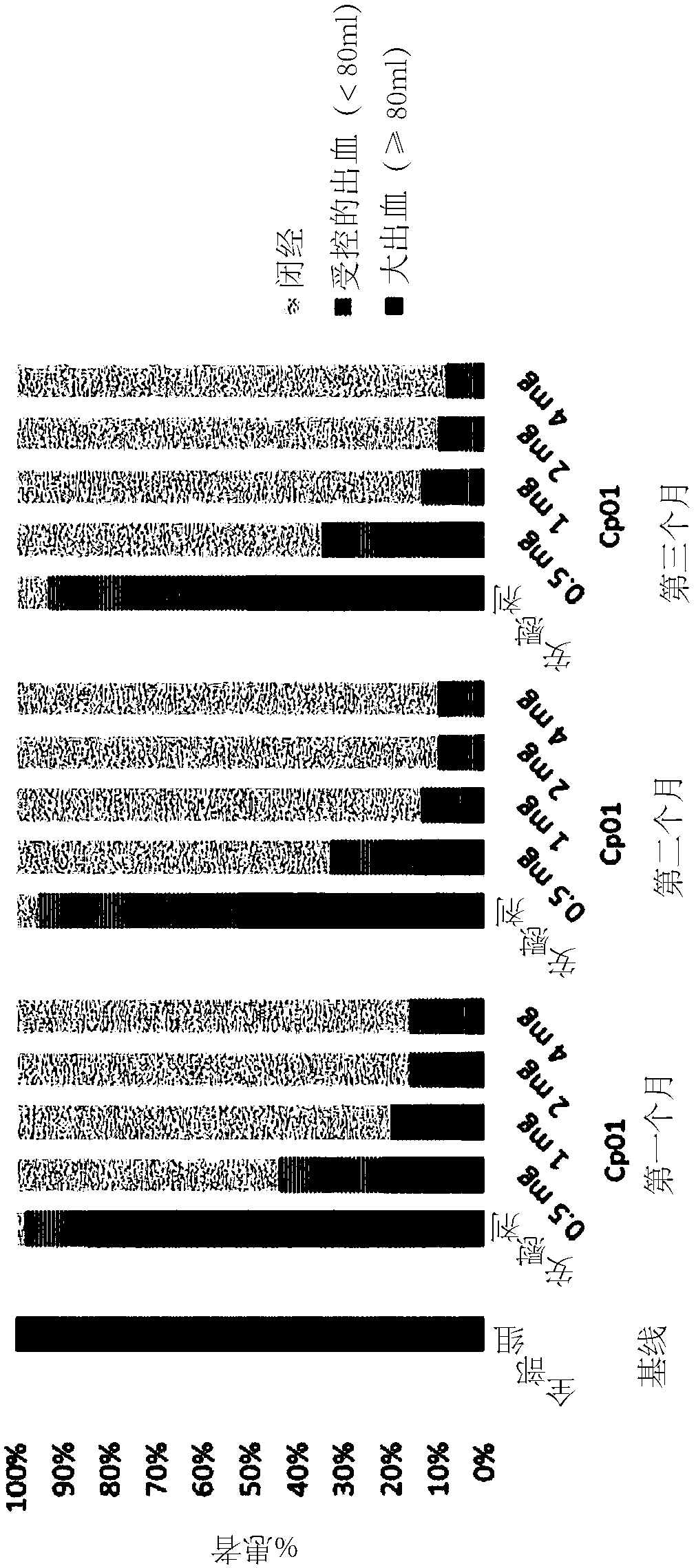
[0131] Females, 18 to 50 years old, with vaginal or abdominal ultrasonography at screening showing uterine fibroids, having at least one uterine fibroid with a maximum diameter ≥ 3.0 cm and heavy menstrual bleeding (HMB) > 80 mL, such women are treated as Subjects participate in research studies. The primary efficacy variable was amenorrhea (yes / no), defined as the absence of monitored bleeding / spotting scheduled after the end of the initial bleeding event until the end of each treatment.
[0132] Treatment groups A1, B1: 30 subjects in each group
[0133] Treatment groups A2, B2: 6 subjects in each group
[0134] A1: Compound 1: 2 mg (12 weeks), Compound 1: 2 mg (12 weeks),
[0135] A2: placebo (12 weeks), compound 1: 2 mg (12 weeks),
[0136] B1: Compound 1: 2 mg (12 weeks), 1 bleeding event, Compound 1: 2 mg (12 weeks),
[0137] • B2: placebo ...
Embodiment 3
[0140] Example 3: Endometrial Thickness and PAEC Using Compound 1 for 3 Months of Treatment
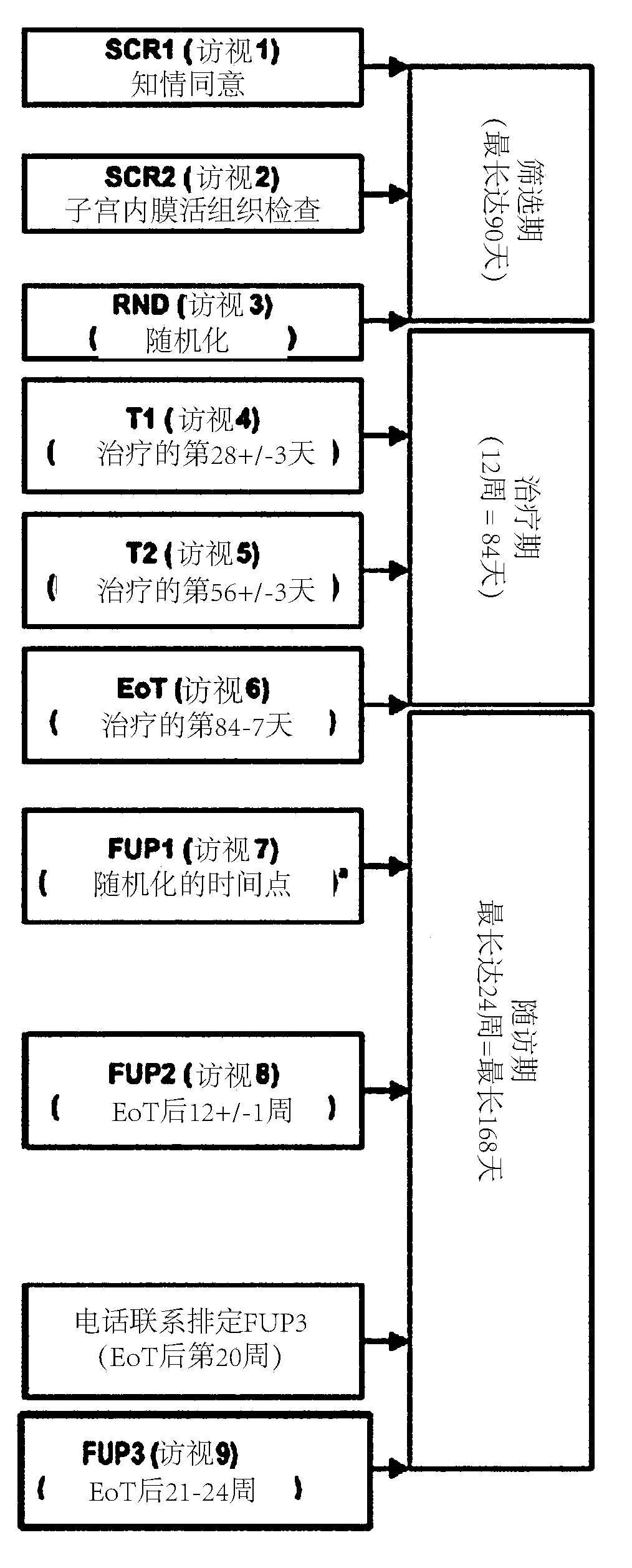
[0141] Randomized, parallel-group, double-blind, placebo-controlled, multicenter study in subjects with uterine fibroids to evaluate different doses of The curative effect of compound 1, in which the primary curative effect variable is endometrial thickness, and the secondary curative effect variable is PAEC.
[0142] Research Protocol (N°15788):
[0143] Test drug: Compound 1
[0144] Dose: 0.5mg, 1mg, 2mg or 4mg, once a day
[0145] Administration route: Oral
[0146] Duration of treatment: 1 x 12 weeks (84 days)
[0147] Comparing drug: placebo
[0148] Duration of treatment: 1 x 12 weeks (84 days)
[0149] Included diagnoses and major criteria:
[0150] Females, 18 to 50 years of age, who have uterine fibroids as a result of transvaginal or abdominal ultrasound at screening, have at least one uterine fibroid with a maximum diameter of 3 cm and heavy menstrual bleeding (HMB) o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com