Process of cloning and further purification to make a recombinant intravenous immunoglobulin
An immunoglobulin, human immunoglobulin technology, applied in immunoglobulin, chemical instruments and methods, peptide/protein components, etc., can solve problems such as precipitation
Inactive Publication Date: 2018-03-27
K 黄
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, a certain percentage of IgG may precipitate into the Fraction III paste
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
[0041] According to one embodiment of the present subject matter,…
[0042] Thanks
[0043] An implementation...
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
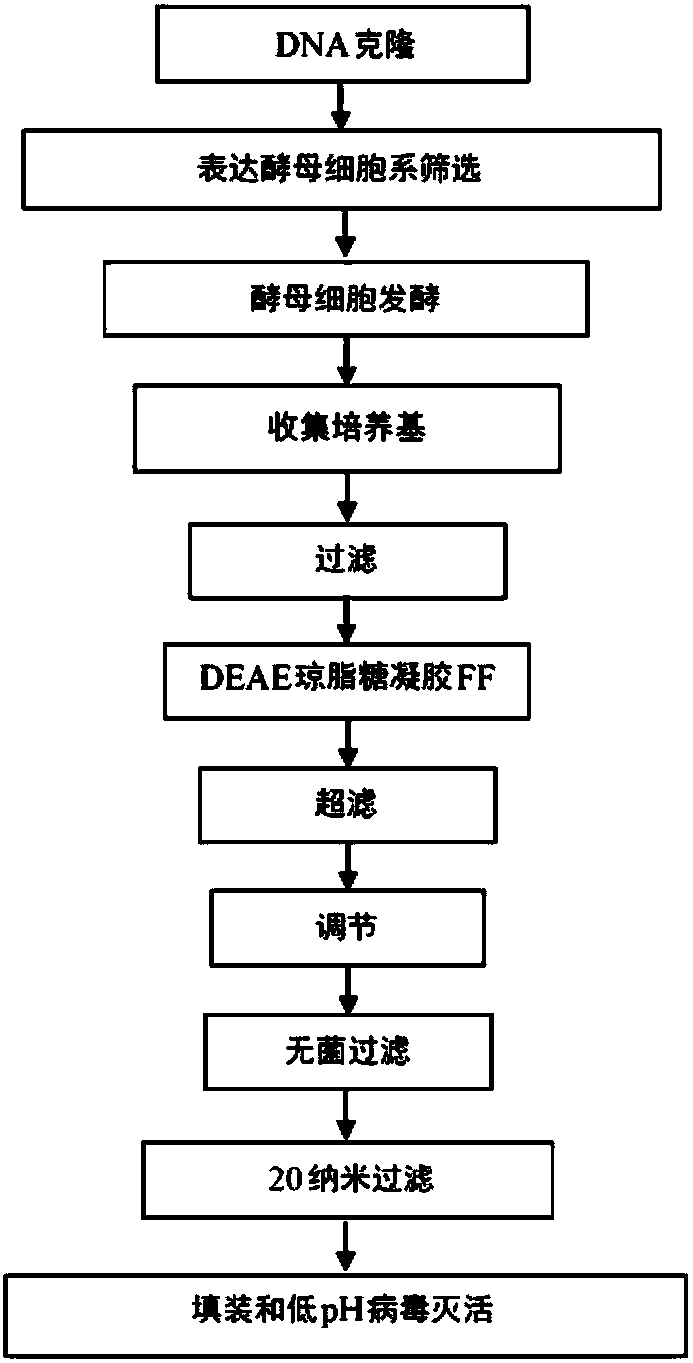
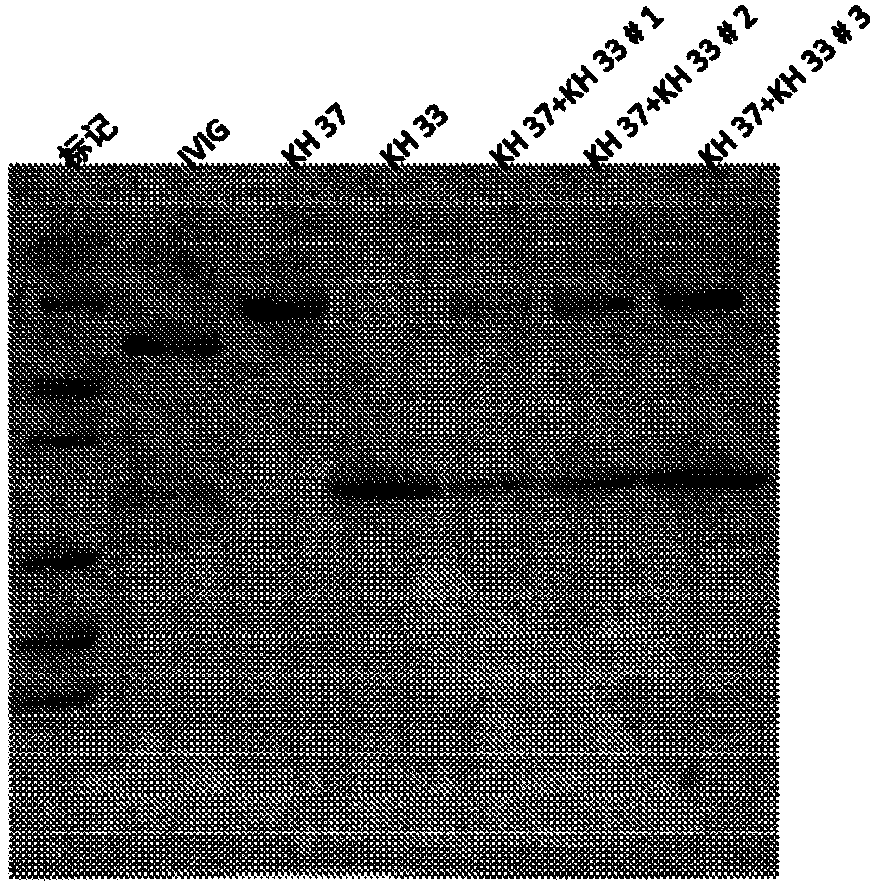
The present subject matter is directed to a process of cloning and purifying recombinant intravenous immunoglobulin (IVIG), comprising cloning a target gene of human immunoglobulin; in vitro screeningof a yeast cell expressing the target gene of human immunoglobulin to create a yeast cell line; fermenting the yeast cell line and collecting a resulting culture medium; filtering the culture medium;undergoing weak anion exchange chromatography to collect a flow-through solution; ultra-filtrating the flow-through solution to reach a desired protein concentration; aseptic filtrating the flow-through solution; nano-filtrating the flow-through solution for virus removal; and filling and incubating the flow-through solution at low pH for virus inactivation to obtain a purified recombinant IVIG.The present subject matter is directed to purified recombinant IVIG having five newly-found proteins, namely KH 33, KH 34, KH 35, KH 36, and KH 37 for both liquid and lyophilized forms.
Description
technical field [0001] This patent application claims priority to US Provisional Patent Application No. 62 / 142,237, filed April 2, 2015, which was filed by the present inventors and is incorporated herein by reference in its entirety. [0002] This subject matter relates to recombinant intravenous immunoglobulin (IVIG) and protein sequences. In particular, the present subject matter relates to methods of cloning and purifying IVIG to produce purified recombinant IVIG in liquid and lyophilized form containing five newly discovered proteins, namely KH 33, KH 34, KH 35, KH 36 and KH 37 . Background technique [0003] Immunoglobulin G (IgG) is an antibody and is a protein complex consisting of four peptide chains, two identical heavy chains and two identical light chains, arranged in typical antibody monomers the Y shape. Each IgG has two antigen binding sites. IgG, representing approximately 75% of human serum antibodies, is the most common type of antibody in the circulati...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K36/899A61K38/17A61K38/38
CPCC07K16/065A61K2039/505B01D61/025B01D61/027B01D61/58C07K16/00B01D2315/10A61K2039/54C07K16/082C07K2317/14
Inventor K·黄
Owner K 黄
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com