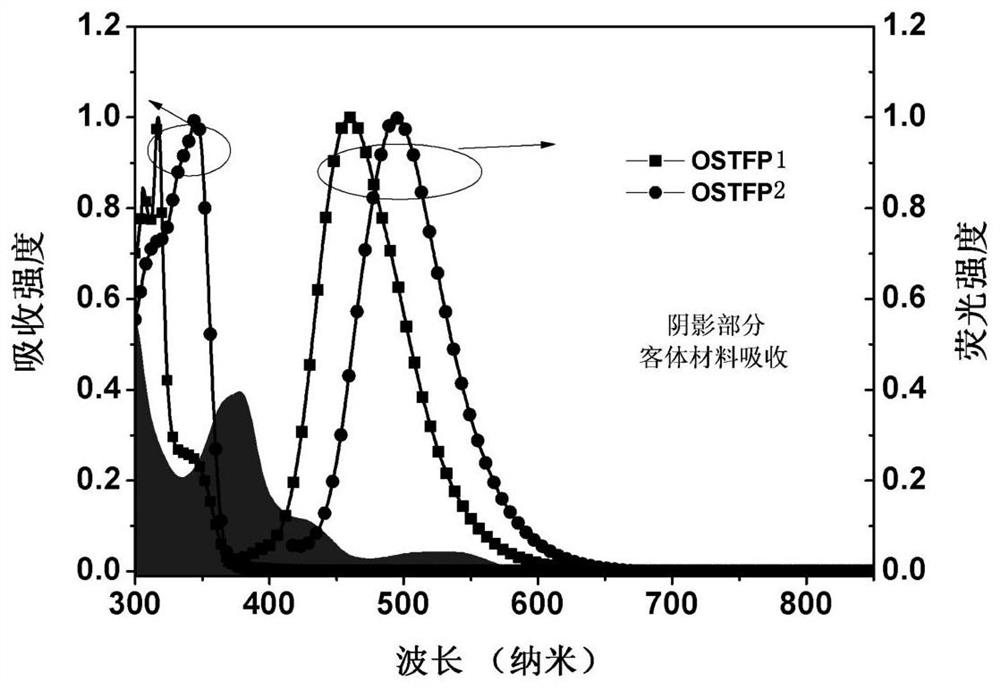
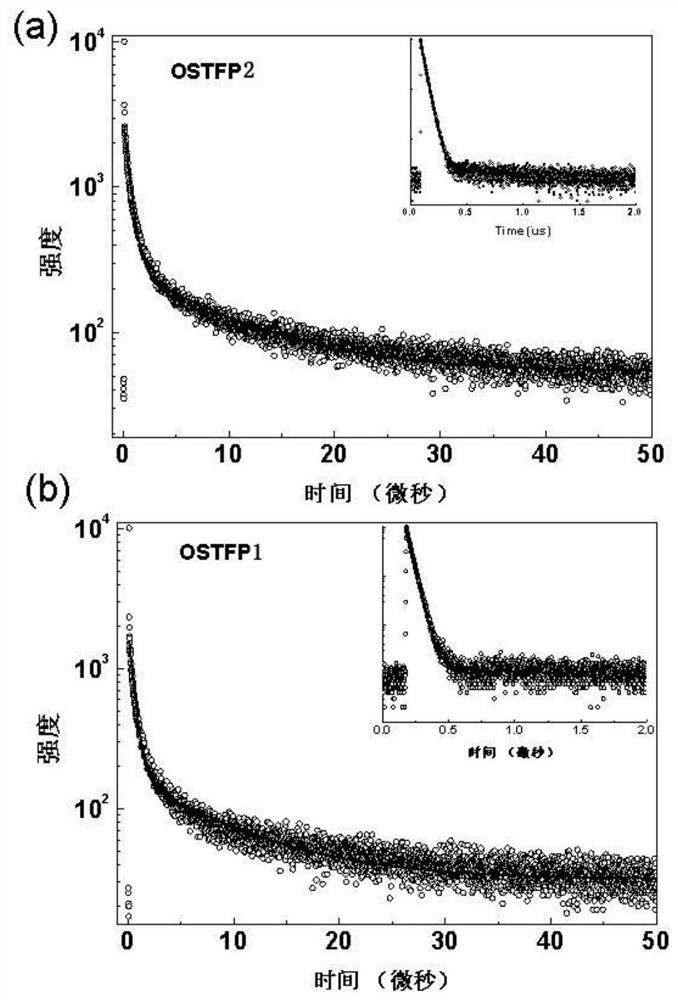
Oxaspirofluorene triphenylamine derivative, preparation method and use thereof
A technology of oxaspirofluorene and triphenylamine, applied in the field of organic optoelectronic materials, can solve problems such as reducing the cost of organic phosphorescent devices, and achieve the effects of good resonance energy transfer and stability, reduction of singlet energy, and high-efficiency electroluminescence performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Step 1: Dissolve 4.23 grams of o-bromoiodobenzene in 80 mL of o-dichlorobenzene under the protection of argon, and add 1.83 grams of phenoxazine, 0.7 grams of cuprous iodide, 0.1 grams of 18-crown 6 ether and 5.0 grams of potassium carbonate in sequence. grams in a 200 ml reaction vial. After reflux for 48 hours under the protection of argon, the reaction solution was cooled to room temperature. The solvent was removed to dryness by a rotary evaporator. The reaction solid was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. The organic layer was dried over anhydrous sodium sulfate and spin-dried. Silica gel was added to spin-dry the obtained solid, which was passed through the column with dichloromethane / petroleum ether=3:7 (volume ratio), and spin-dried to obtain 3.04 g of 2-bromooxatriphenylamine, with a yield of 90%. Step 2: Dissolve 1.52 g of 2-bromooxatriphenylamine in 80 mL of tetrahydrofuran under the pro...
Embodiment 2
[0050] Step 1: Same as Step 1 of Example 1.
[0051] Step 2: Same as Step 2 of Example 1.
[0052] Step 3: Dissolve 2.0 g of 2-bromooxaspirofluorenetriphenylamine in 80 mL of tetrahydrofuran under the protection of argon, cool to -78°C, and slowly add 2.0 mL of n-butyllithium into the solution through a constant pressure dropping funnel, React for 1 hour. Then 1.1 g of bis(trimethylphenyl)boron fluoride was dissolved in 40 mL of tetrahydrofuran under the protection of argon and added dropwise to the reaction solution. After 2 hours of reaction at low temperature, it was gradually raised to room temperature. After 12 hours of reaction, 5 mL of water was added to the reaction, and then the solvent was spin-dried under reduced pressure. The solid was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. The organic layer was dried over anhydrous sodium sulfate and spin-dried. Pass through the column with dichloromethane / petro...
Embodiment 3
[0054] Step 1: Same as Step 1 of Example 1.
[0055] Step 2: Same as Step 2 of Example 1.
[0056] Step 3: Dissolve 1.52 g of 2-bromooxatriphenylamine in 80 mL of tetrahydrofuran under the protection of argon, cool to -78°C, slowly add 2.38 mL of n-butyllithium into the solution through a constant pressure dropping funnel, and react 1 Hour. Then 1.3 g of 3-bromofluorenone was dissolved in 40 mL of tetrahydrofuran under the protection of argon and added dropwise to the reaction solution. After 1 hour of reaction at low temperature, it was gradually raised to room temperature. After 12 hours of reaction, 5 mL of water was added to the reaction, and then the solvent was spin-dried under reduced pressure. The solid was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. The organic layer was dried over anhydrous sodium sulfate and spin-dried. The solid obtained by spin-drying was dissolved in 45 mL of glacial acetic acid and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com