Pharmaceutical compositions useful for the treatment of tissue injury
A technology of tissue damage and composition, which is applied in the field of pharmaceutical composition for the treatment of tissue damage, and can solve the problems of discomfort and increased risk of specimens, and weakened effects of treatment plans, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、2
[0129] Embodiment 1,2 materials and methods:
[0130] Injectable AF Compositions
[0131] Reagent : AMD3100 with tacrolimus (powder) was obtained from Sigma-Aldrich (St. Louis, MO).
[0132] tacrolimus preparation : Due to the hydrophobicity of tacrolimus powder and its poor solubility in aqueous solution (such as normal saline), tacrolimus powder is dissolved in 100% ethanol (8% of the total volume), castor oil (2% of the total volume) %) and sterile saline for injection (90% of the total volume). The experimental formula of tacrolimus is C 44 h 69 NO 12 •H 2 O, the relative molecular weight is 822.03.
[0133] AMD3100 formulation : 24 mg AMD3100 dissolved in sterile water containing 5.9 mg sodium chloride, pH adjusted to 6.0-7.5 with hydrochloric acid and, if necessary, sodium hydroxide. The molecular weight of AMD3100 is 502.79 g / mol.
[0134] AF composition : Add dissolved tacrolimus to AMD3100 solution, the weight ratio of tacrolimus to AMD3100 is abou...
Embodiment 1
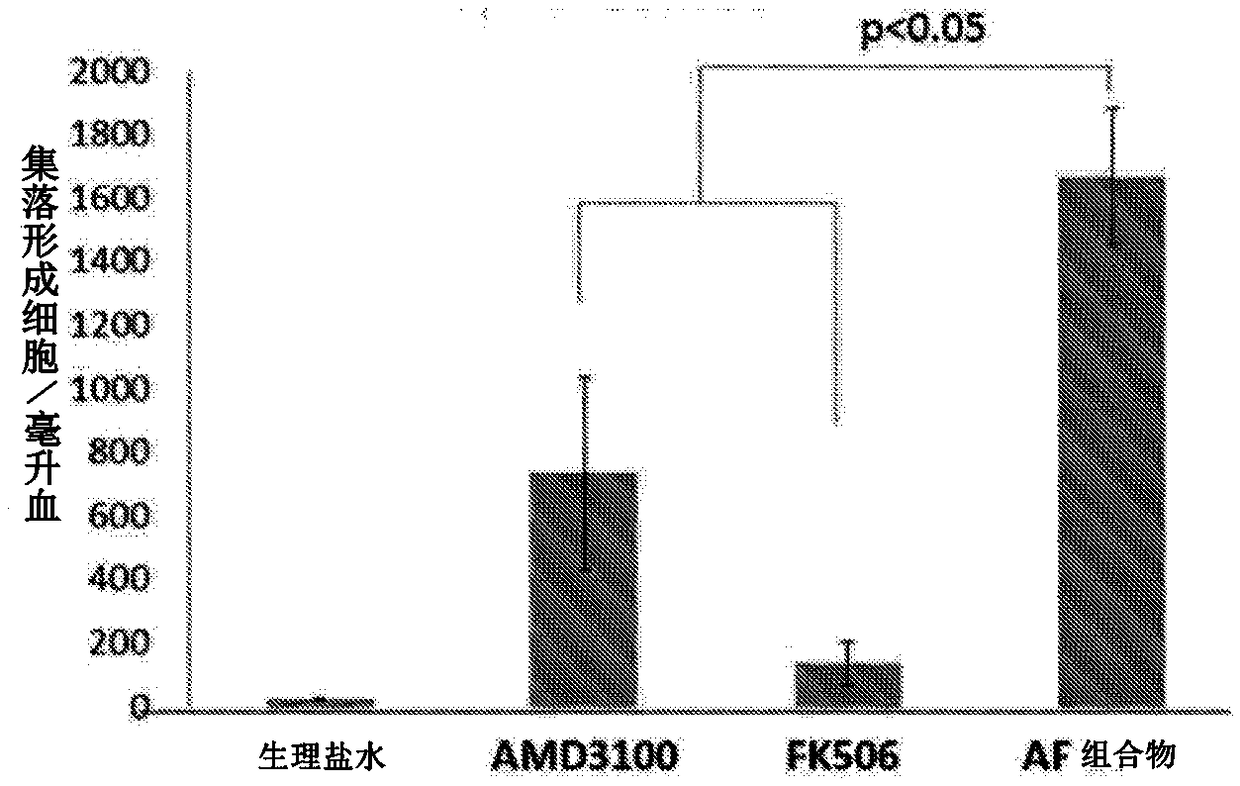
[0135] Example 1 - Stem cell mobilization activity test of AF composition
[0136] Hematopoietic colony-forming cell (CFC) assays have been widely used in research and clinical applications in human and animal models to quantify and evaluate the content of hematopoietic progenitor cells in cell samples. The stem cell mobilization activity can be detected by CFC test of the mobilized peripheral blood samples. CFCs can divide and differentiate into more mature populations of cells detectable by light microscopy. This allows quantification of pluripotent stem cell lineages mobilized by pharmaceutical agents.
[0137] C57 / B6 mice were divided into 4 treatment groups: 1) control group treated with normal saline; 2) AMD3100 group (1.0 mg / kg); 3) tacrolimus treated with low dose (0.1 mg / kg) Group; 4) AF composition (including AMD3100 and tacrolimus). Animals were dissected 3 hours after drug treatment, peripheral blood was collected, and peripheral blood mononuclear cells (PBMCs...
Embodiment 2
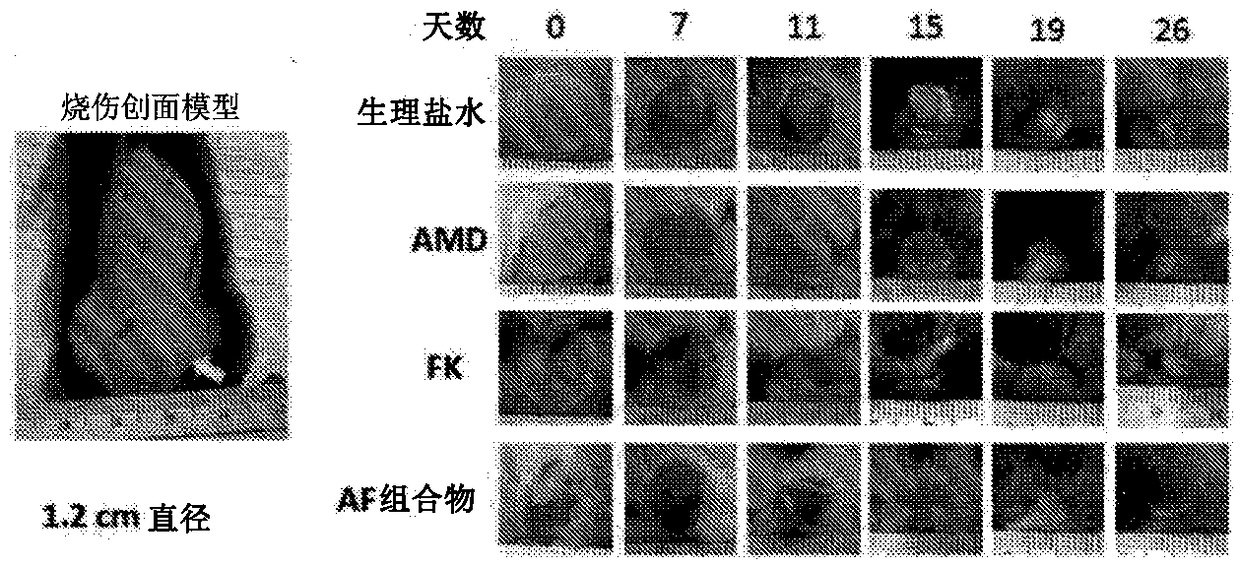
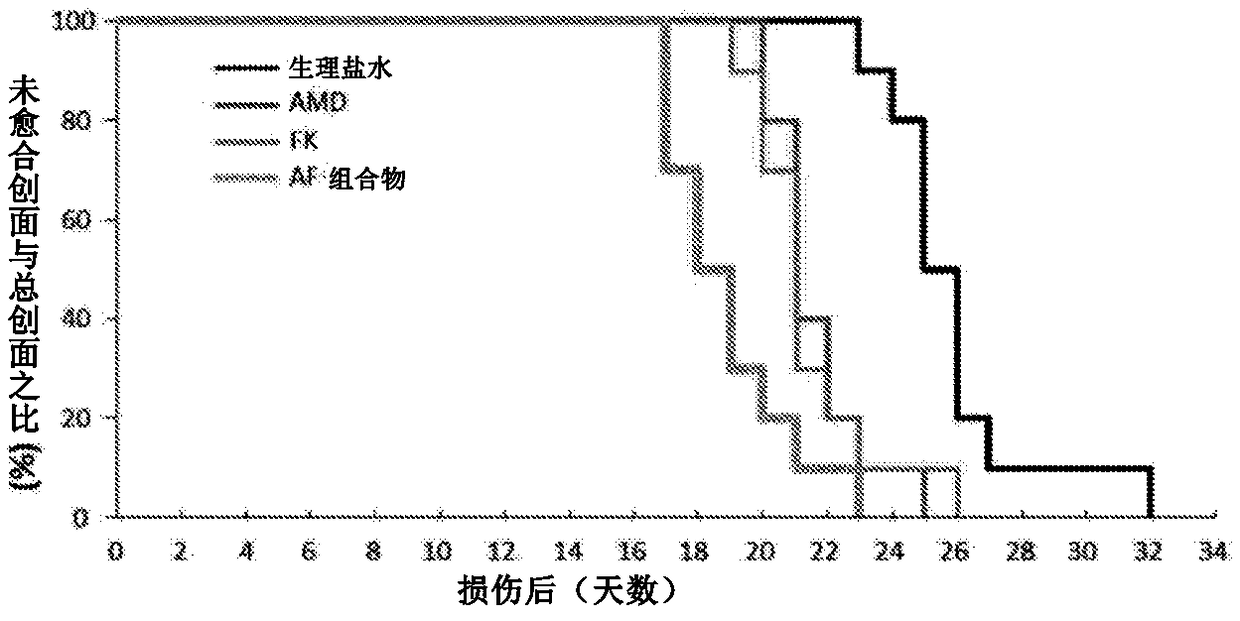
[0139] Example 2 - AF composition promotes burn wound healing in mice
[0140] Stem cell therapy can improve the quality of burn wound healing, reduce scar formation, and rebuild the skin. To avoid the costly and time-consuming preparation of hematopoietic stem cells required in the treatment of burns, this study shows that endogenous bone marrow stem cells can be pharmacologically mobilized by AF combinations to treat burns.
[0141] C57 / B6 mouse back skin caused full-thickness burns (diameter 12mm) ( figure 2 ). Burned mice were randomly divided into 4 experimental groups as follows, and received subcutaneous injection of normal saline or drugs immediately after the wound surface was formed until healing: (1) the control group treated with normal saline; (2) treated every other day (1.0 mg / kg ) AMD3100 group; (3) FK506 group treated with low dose (0.1 mg / kg) every day; and (4) AF composition group treated every other day. All wound assessments were double-blind.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com