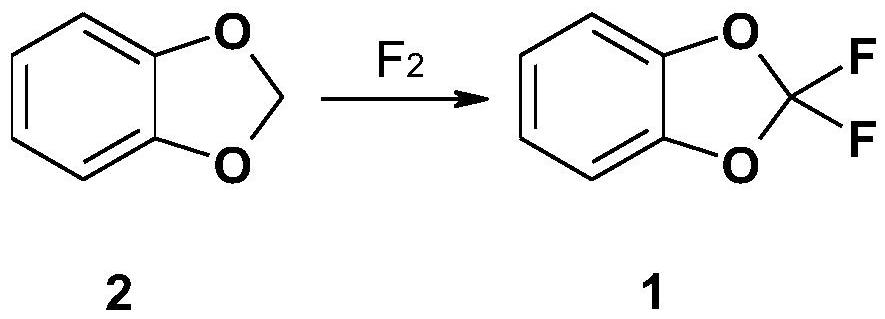
A kind of preparation method of difluoropiperone
A difluoro piperonyl ring and piperonyl ring technology, applied in the direction of organic chemistry and the like, can solve the problems of difficult recycling of sulfolane, poor atom economy, long reaction time, etc., and achieve good heat exchange efficiency, low pollution, and safe and controllable reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh 100g of piperonylcycline and 900g of acetonitrile, mix and prepare a 10% piperonine in acetonitrile solution, then add 1g of trifluoroacetic acid, mix well and set aside.
[0022] Adjust the flow rate of fluorine gas and nitrogen gas to prepare a mixed gas of fluorine gas and nitrogen gas with a mass fraction of 10% for use.
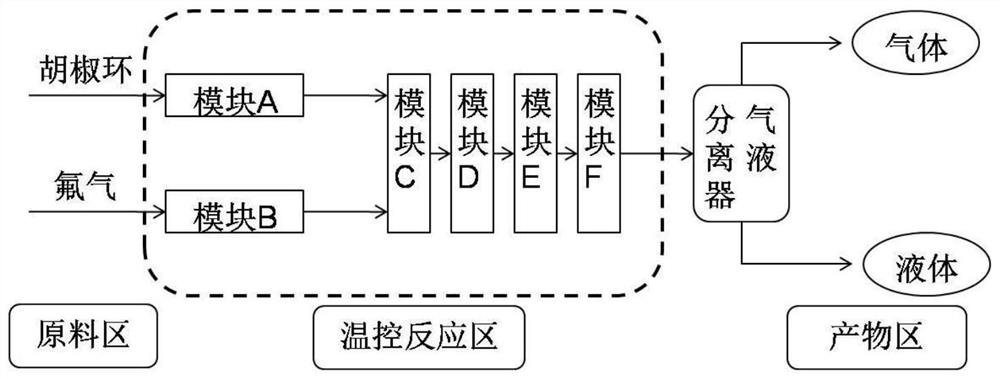
[0023] use as figure 1 In the reaction process shown, the microchannel reactor made of silicon carbide is selected, the liquid holding capacity of each module is 1ml, the reaction temperature of the module is set at -20°C, the pumping rate is controlled to adjust the reaction residence time, and the residence time is set at 20s , the A module is continuously and uniformly pumped into the piperonine acetonitrile solution (containing 1% trifluoroacetic acid) with a mass fraction of 10% for precooling, while the B module is continuously and uniformly fed with a mass fraction of 10% fluorine-nitrogen mixed gas, and its fluorine The molar amount...
Embodiment 2
[0025] Weigh 150g of piperonylcycline and 850g of acetonitrile, mix and prepare a 10% piperonine in acetonitrile solution, then add 1g of trifluoromethanesulfonic acid, mix well and set aside.
[0026] Adjust the flow rate of fluorine gas and nitrogen gas to prepare a mixed gas of fluorine gas and nitrogen gas with a mass fraction of 50% for use.
[0027] use as figure 1 In the reaction process shown, the microchannel reactor made of silicon carbide is selected, the liquid holding capacity of each module is 1ml, the reaction temperature of the module is set at -40°C, the pumping rate is controlled to adjust the reaction residence time, and the residence time is set at 10s , A module is continuously and uniformly pumped into a piperonine acetonitrile solution (containing 1% trifluoromethanesulfonic acid) with a mass fraction of 15% for precooling, while the B module is continuously and uniformly fed with a mass fraction of 50% fluorine-nitrogen mixed gas, The molar amount of f...
Embodiment 3
[0029] Weigh 100 g of piperonylcycline and 900 g of formic acid, mix and prepare a piperonylcycline formic acid solution with a mass fraction of 10%, then add 0.5 g of trifluoromethanesulfonic acid, mix well and set aside.
[0030] Adjust the flow rate of fluorine gas and nitrogen gas to prepare a mixed gas of fluorine gas and nitrogen gas with a mass fraction of 10% for use.
[0031] use as figure 1 In the reaction process shown, the microchannel reactor made of silicon carbide is selected, the liquid holding capacity of each module is 1ml, the reaction temperature of the module is set at -10°C, the pumping rate is controlled to adjust the reaction residence time, and the residence time is set at 30s , A module is continuously and uniformly pumped into a piperonine solution (containing 0.5% trifluoromethanesulfonic acid) with a mass fraction of 10% for precooling, while the B module is continuously and uniformly fed with a mass fraction of 10% fluorine-nitrogen mixed gas, Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com