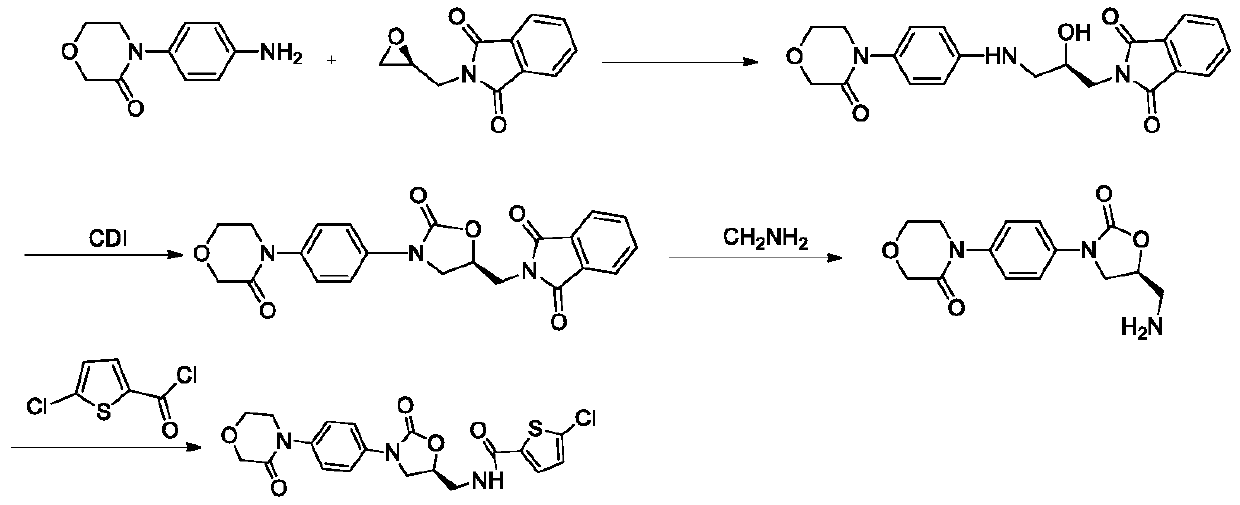
A kind of preparation method of rivaroxaban intermediate
A technology for rivaroxaban and intermediates, applied in the field of preparation of rivaroxaban intermediate S-N-glycidyl phthalimide, which can solve the problems of difficult purification of mixtures, difficult industrialization, and harsh reaction conditions , to achieve the effect of avoiding special requirements of equipment, reducing production cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
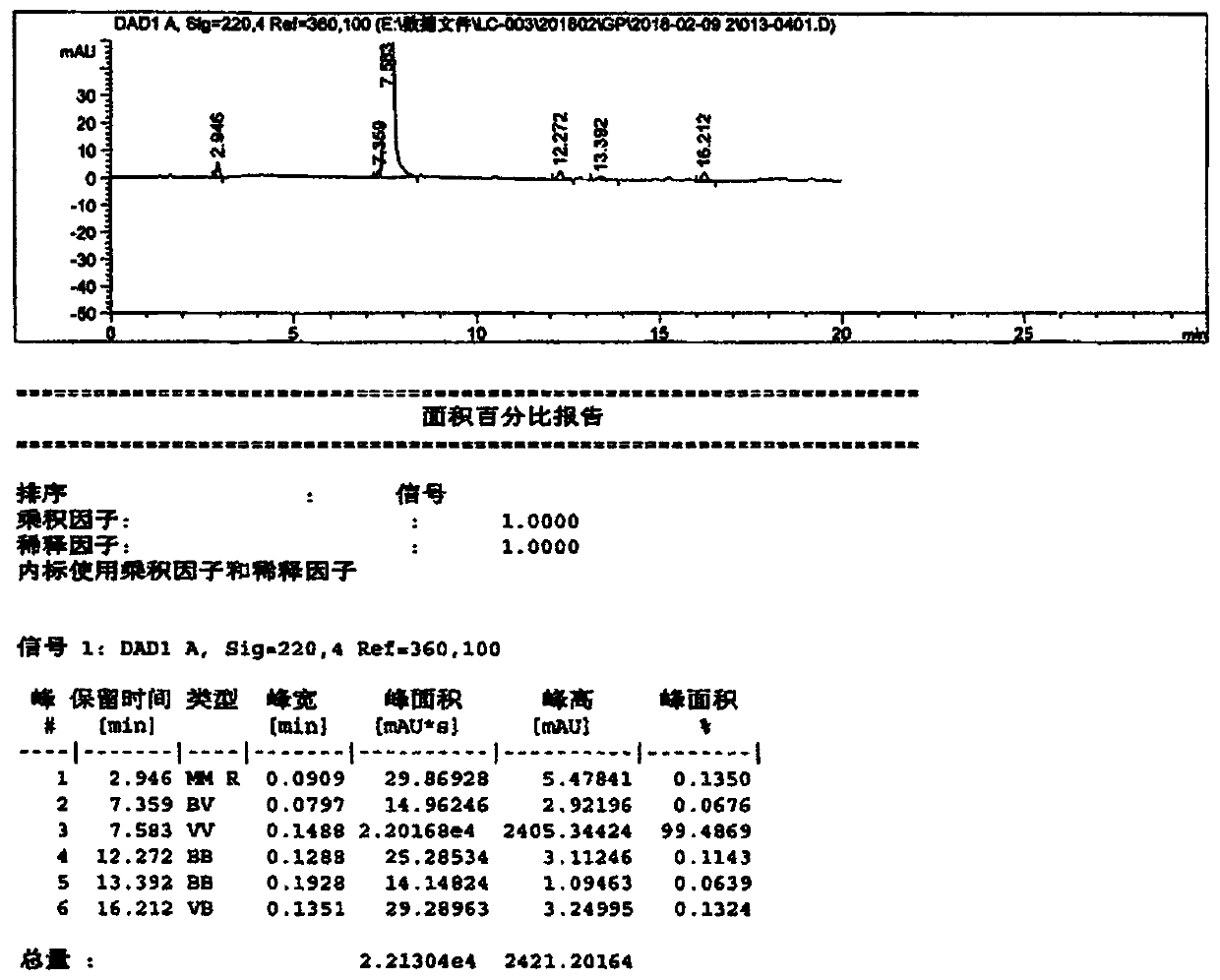
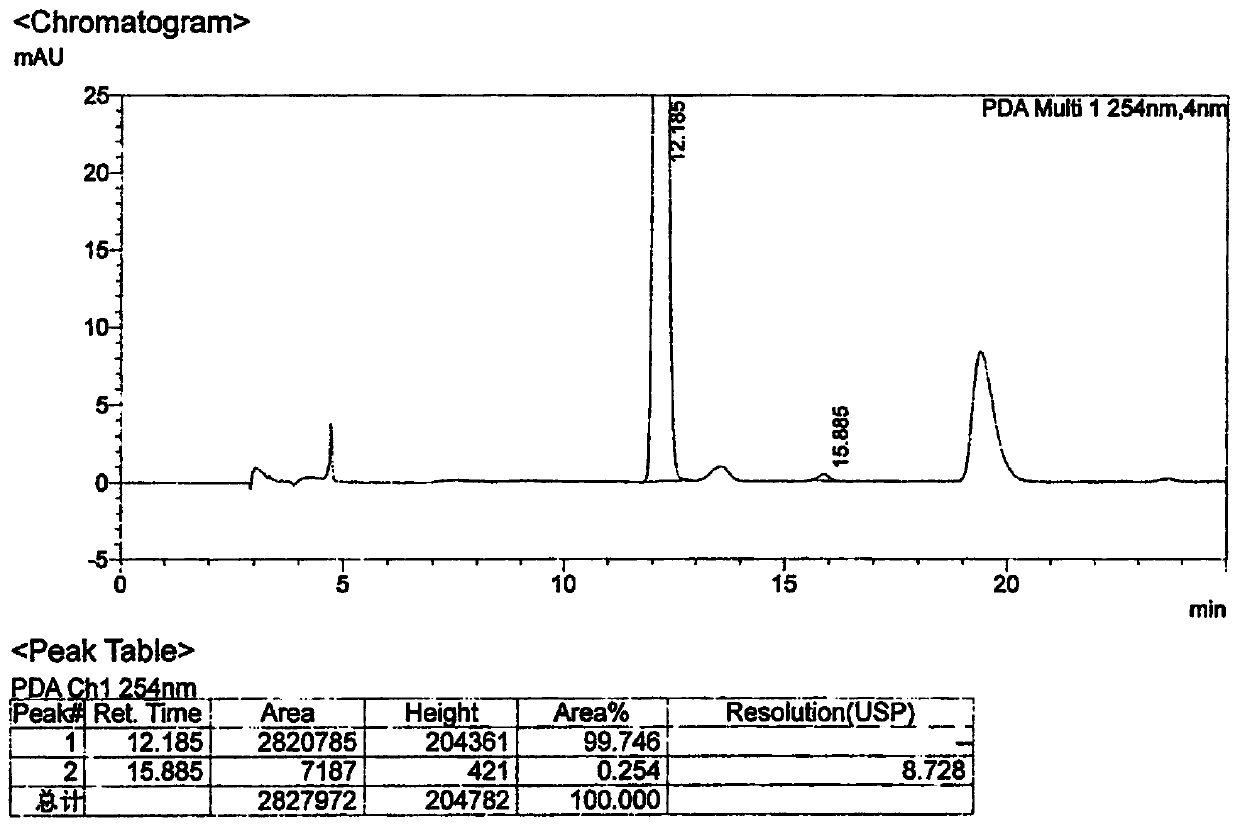
Image
Examples
Embodiment 1
[0062] Example 1: Preparation of 2-((S)-3-chloro-2-hydroxypropyl)isoindoline-1,3-dione (Compound I)
[0063]
[0064] Put phthalimide (100.0 g 0.68 mol), 1.0 g of basic alumina, 1.0 g of TMBAC, 300 g of isopropanol, and 23.0 g of S-epichlorohydrin into a four-necked flask, and stir. Control the inner temperature at 20-25°C and keep it warm for 12 hours, take samples and control it until the residue of phthalimide in the reaction solution is ≤1.0%, and the reaction ends. Concentrate to remove isopropanol, add 400g of toluene to dissolve and filter to recover alumina for use; the filtrate is washed with 2×100g of water, concentrated, and 450g of n-heptane is added to crystallize to obtain 150g of compound I (yield 92.1%).
Embodiment 2
[0065] Example 2: Preparation of 2-((S)-3-chloro-2-hydroxypropyl)isoindoline-1,3-dione (Compound I)
[0066]
[0067] Put phthalimide (100.0g 0.8mol), basic aluminum oxide (recovered from Example 1) 1.0g, TMBAC1.0g, isopropanol 300g, S-epichlorohydrin 23.0g into four ports bottle, stir. Control the inner temperature at 20-25°C and keep it warm for 12 hours, take samples and control it until the residue of phthalimide in the reaction solution is ≤1.0%, and the reaction ends. Concentrate to remove isopropanol, add 400g of toluene to dissolve and filter to recover alumina for use; the filtrate is washed with 2×100g of water, concentrated, and 450g of n-heptane is added to crystallize to obtain 149.4g of compound I (91.7% yield).
Embodiment 3
[0068] Example 3: Preparation of 2-((S)-3-chloro-2-hydroxypropyl)isoindoline-1,3-dione (Compound I)
[0069]
[0070] Put phthalimide (100.0g 0.8mol), basic aluminum oxide (recovered from Example 2) 1.0g, TMBAC1.0g, isopropanol 300g, S-epichlorohydrin 23.0g into four ports bottle, stir. Control the inner temperature at 20-25°C and keep it warm for 12 hours, take samples and control it until the residue of phthalimide in the reaction solution is ≤1.0%, and the reaction ends. Concentrate to remove isopropanol, add 400g of toluene to dissolve and filter to recover alumina for use; the filtrate is washed with 2×100g of water, concentrated, and 450g of n-heptane is added to crystallize to obtain 149.0g of compound I (yield 91.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com