Micromolecular saccharide-containing system for in-vitro high-efficiency protein synthesis
A small molecule sugar and protein technology, applied in the field of efficient protein synthesis system, to achieve the effect of simple and fast method, increase yield, and meet research requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0031] Example 7
[0032] Preparation of cell disruption products:
[0033] The cultured Escherichia coli (Escherichia coli BL21(DE3) CICC 23796, purchased from CICC) was collected, suspended, centrifuged, mechanically broken, and stored.
[0034] In addition to mechanical methods, cell disruption methods can also be repeated freezing and thawing methods, ultrasonic treatment methods, enzymatic methods, alkaline lysis methods or chemical permeation methods.
[0035] The crushing process uses an ultrasonic cell pulverizer with a power of 20W, ultrasonic for 5s per cycle, 10s stop, and a total time of 1h (10-15mL is taken each time for crushing).
[0036] Examples 8-10 are composition 1, see Table 3.
[0037] table 3
[0038]
[0039] Examples 11-13 are amino acid aqueous solutions, see Table 4
[0040] Table 4
[0041]
[0042] Examples 14-16 are reaction buffers, see Table 5
[0043] table 5
[0044]
[0045] Examples 17-19 are energy supplement liquids, see Table 6
[0046]
Example Embodiment
[0047] Examples 20-22 are composition 2 (each component is volume ratio), see Table 7
[0048]
Example Embodiment
[0049] Example 23
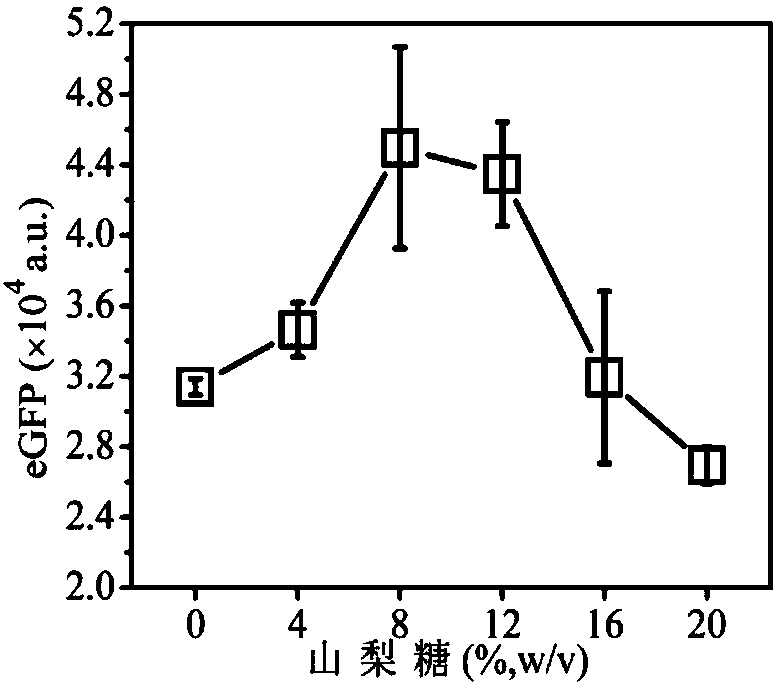
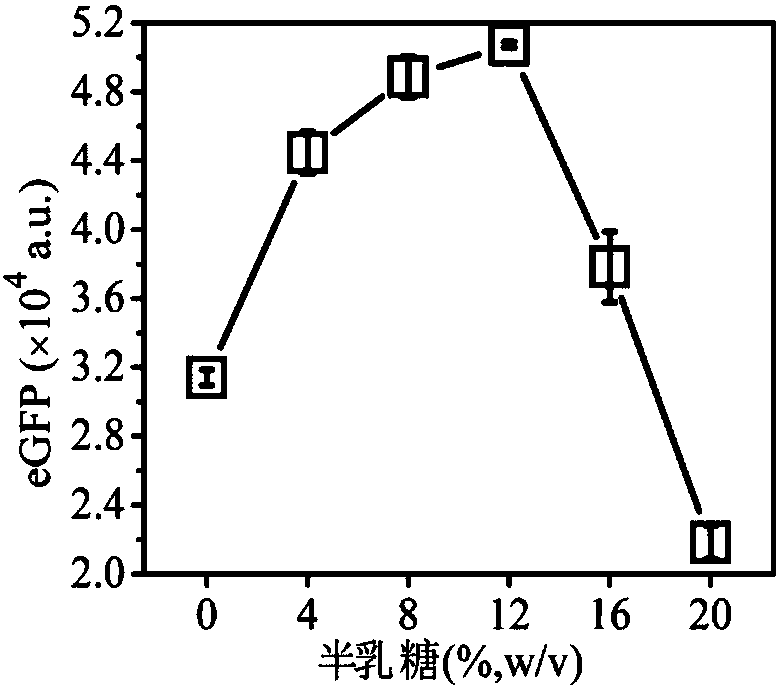
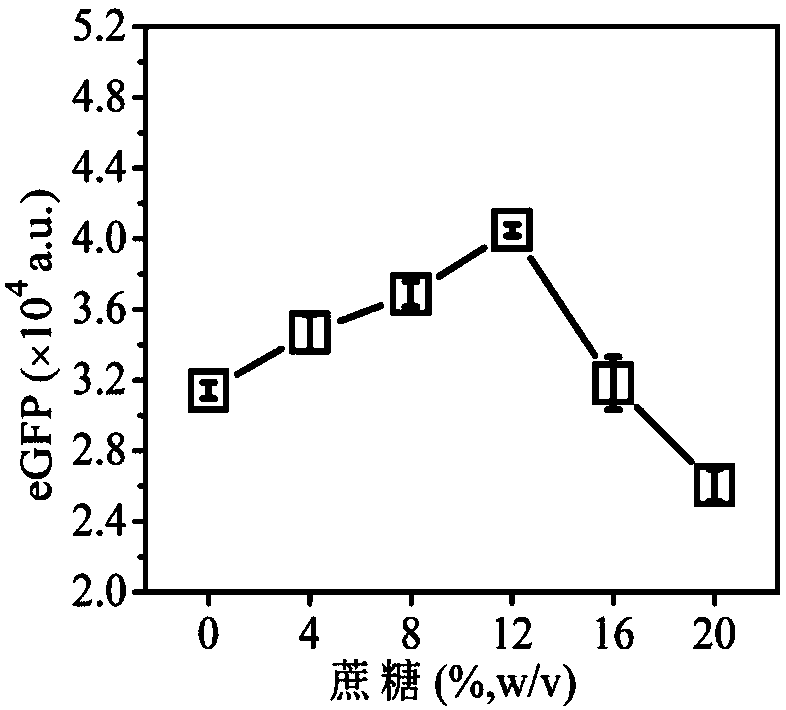
[0050] A system for efficiently synthesizing proteins in vitro containing small molecular sugars, with a volume ratio of 20:25:1: (1.5, 3, 4.5, 6, 7.5), and the ratio includes composition 1 (prepared in Example 8) and composition 2 (Prepared in Example 20), gene (pRset-eGFP (purchased from: Promega Corporation)) and 1.334g / ml sorbose aqueous solution;
[0051] The preparation process is: mix composition 1, composition 2, gene and 5 volumes of 1.334g / ml sorbose aqueous solution, put them in a 96-well plate, react at 30°C for 12 hours, and perform detection to obtain protein expression The amount varies with the concentration of sorbose as follows figure 1 Shown.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap