Carvedilol immediate release formulation having improved madescent
An immediate-release preparation and preparation technology, applied in the field of carvedilol immediate-release preparations, can solve the problems of surface swelling, easy rupture, cracked tablets, etc., and achieve high stability, unchanged appearance, and excellent immediate-release characteristics. Effect
Inactive Publication Date: 2018-09-28
SAMJIN PHARMA
View PDF9 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
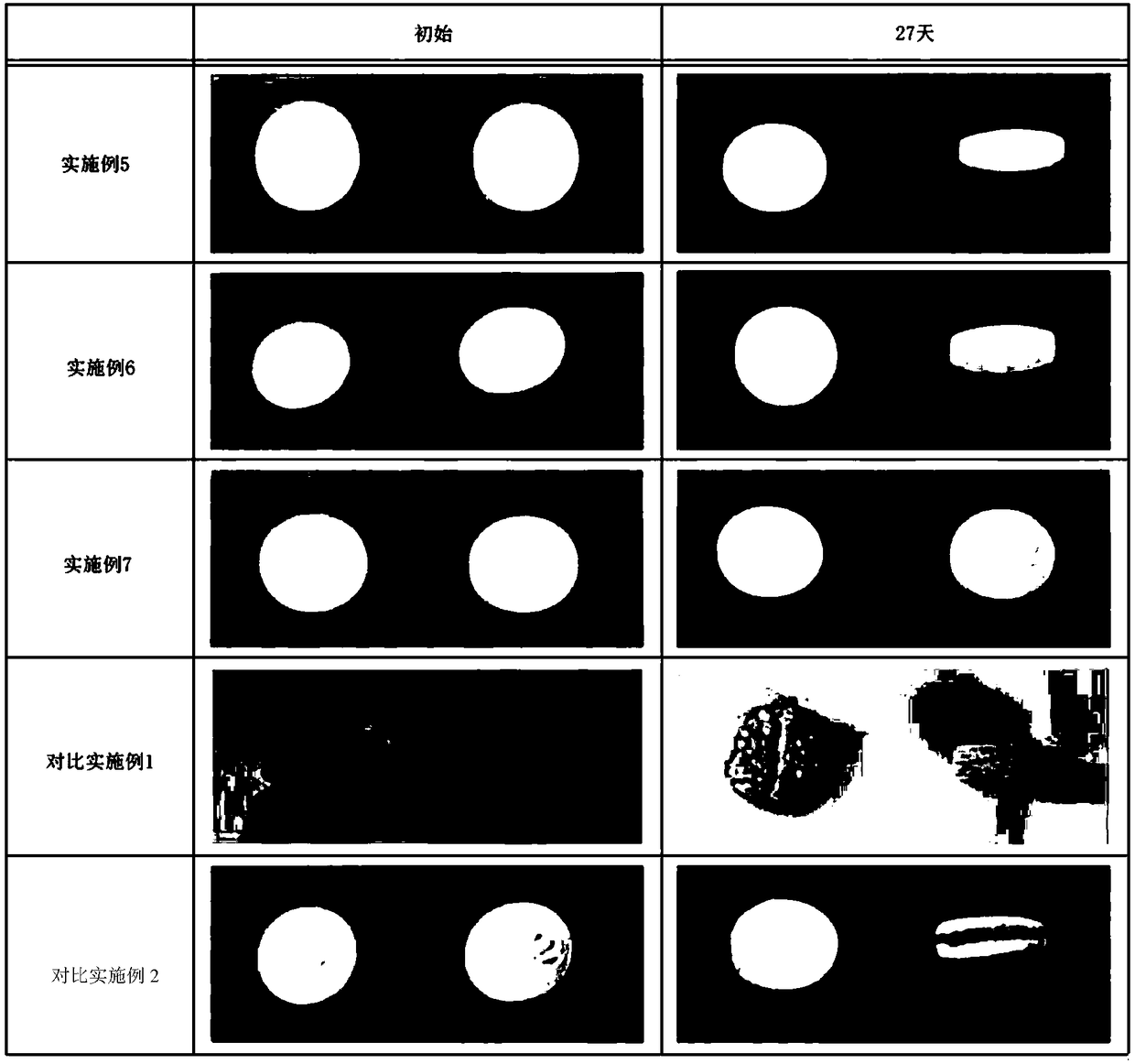
[0009] However, in many cases in clinical use carvedilol tablets are prescribed and stored with the aluminum foil blister (Alu-Alu) pack removed, and unpacked tablets may be problematic because the formulation The appearance of the tablet may change, including surface swelling, cracked sides, or the tablet is prone to breakage
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1-8
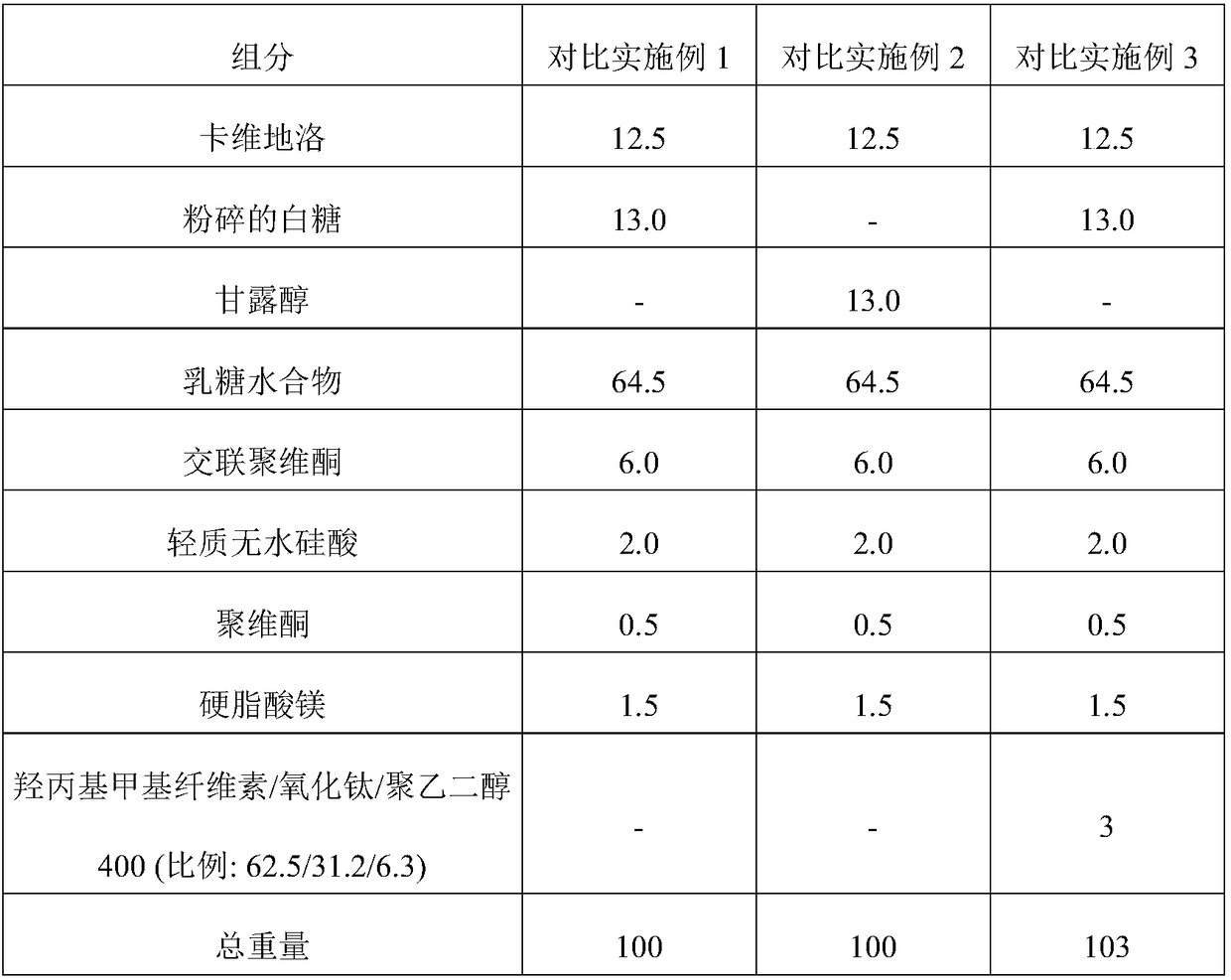
[0060] The uncoated tablet of comparative example 1-2 is coated with the coating dispersion liquid comprising the components shown in the following table 2 according to the corresponding film coating ratio (%) to prepare the tablet of embodiment 1-8. Coated tablet.
[0061] Table 2
[0062] (unit: mg)
[0063]
[0064]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
Disclosed is an immediate-release carvedilol formulation, which is configured such that a coating layer including a polymer, wax, fatty acid and / or fatty acid ester is formed on the surface thereof. This formulation has improved madescent, and thus can exhibit high stability, in which the initial appearance of the formulation can be maintained without cracking or breaking even under storage conditions at high relative humidity.
Description
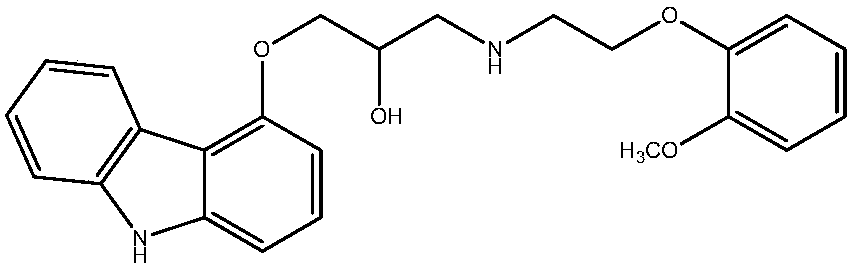
technical field [0001] The present invention relates to an immediate release formulation of carvedilol with improved hygroscopicity. Background technique [0002] Carvedilol is used in the treatment of hypertension and its chemical name is "1-(9H-carbazol-4-yloxy)-3-[[2-(2-ketooxyphenoxy)ethyl]amino ]-2-propanol ", the structure is shown in the following chemical formula I. [0003] [chemical formula I] [0004] [0005] Carvedilol dilates blood vessels by blocking α1 and β. As the only third-generation β-blocker, it can be used for hypertension, angina pectoris, heart failure, etc. Carvedilol is very effective in lowering blood pressure without causing side effects such as edema, reflex tachycardia, and dry cough that are often caused by other antihypertensive drugs. Carvedilol was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of high blood pressure, specifically congestive heart failure. [0006] Carvedilol is somewhat insoluble, so...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/28A61K31/403A61K47/44A61K9/48A61K47/32A61K47/34A61K47/38
CPCA61K31/403A61K9/284A61K9/2866A61K31/404A61P9/12A61K9/0002A61K9/2013A61K9/282A61K9/288
Inventor 曹义焕崔承柱李成宇申熙钟奇旻孝崔美花吴泰勋
Owner SAMJIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com