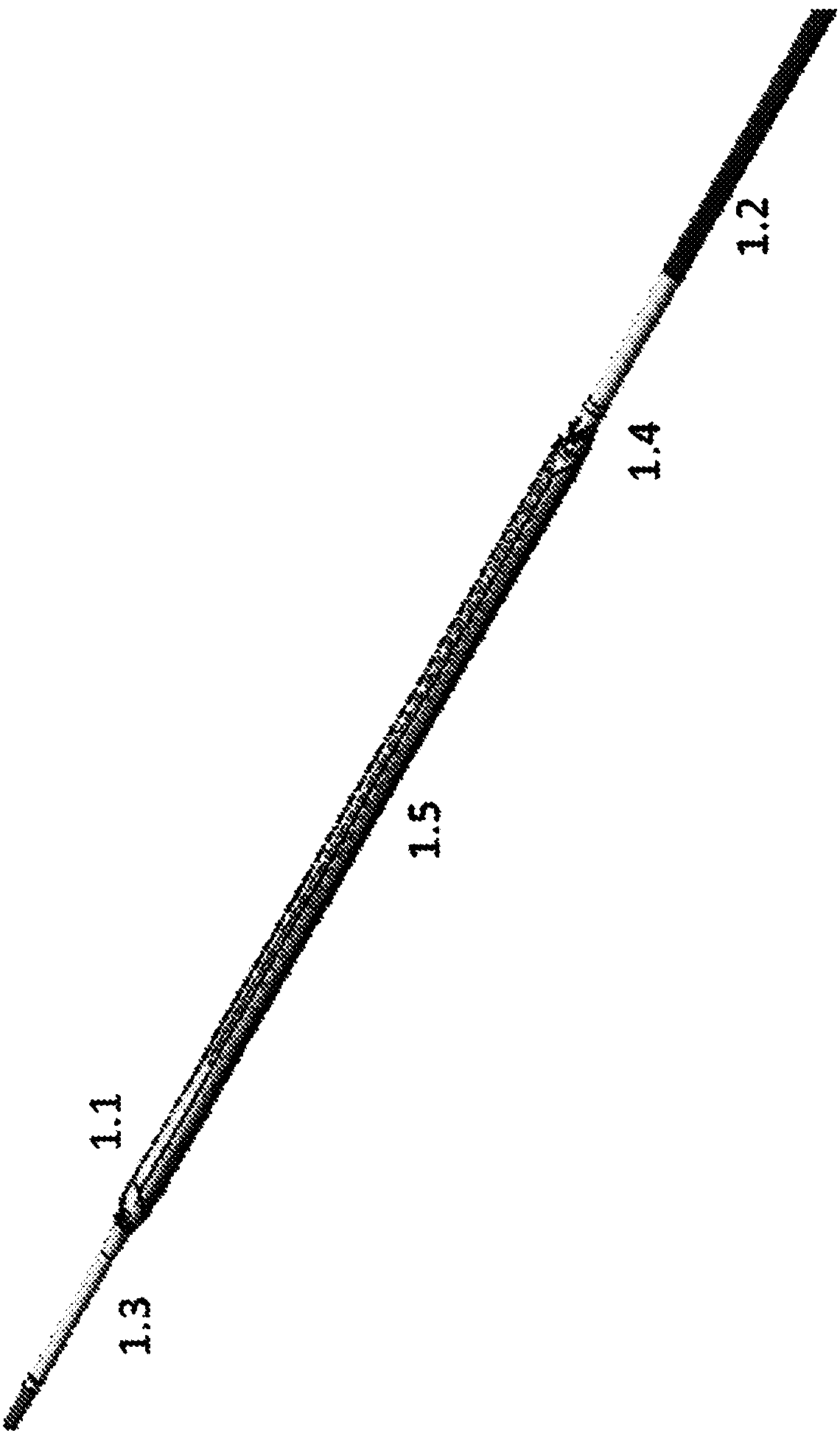
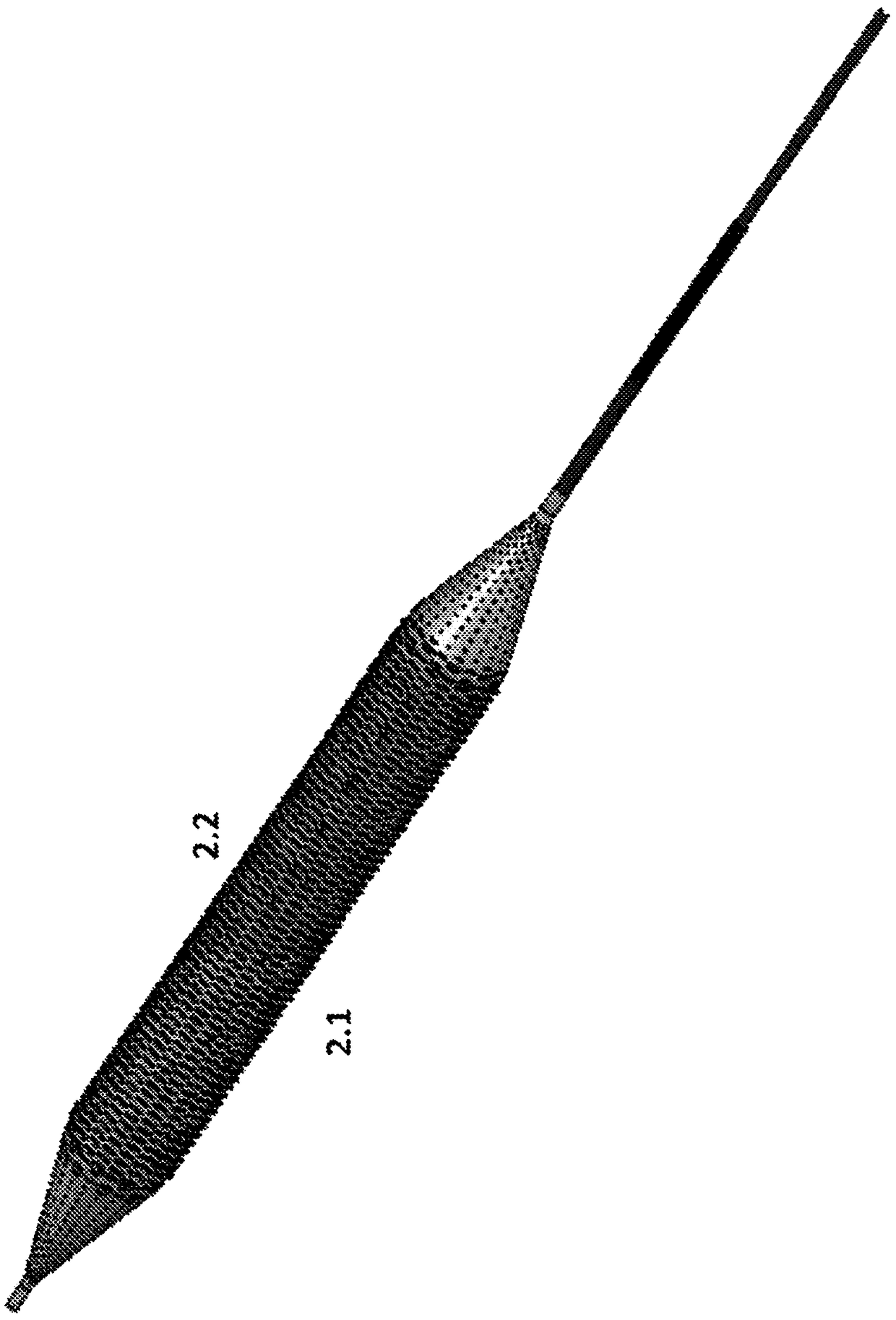
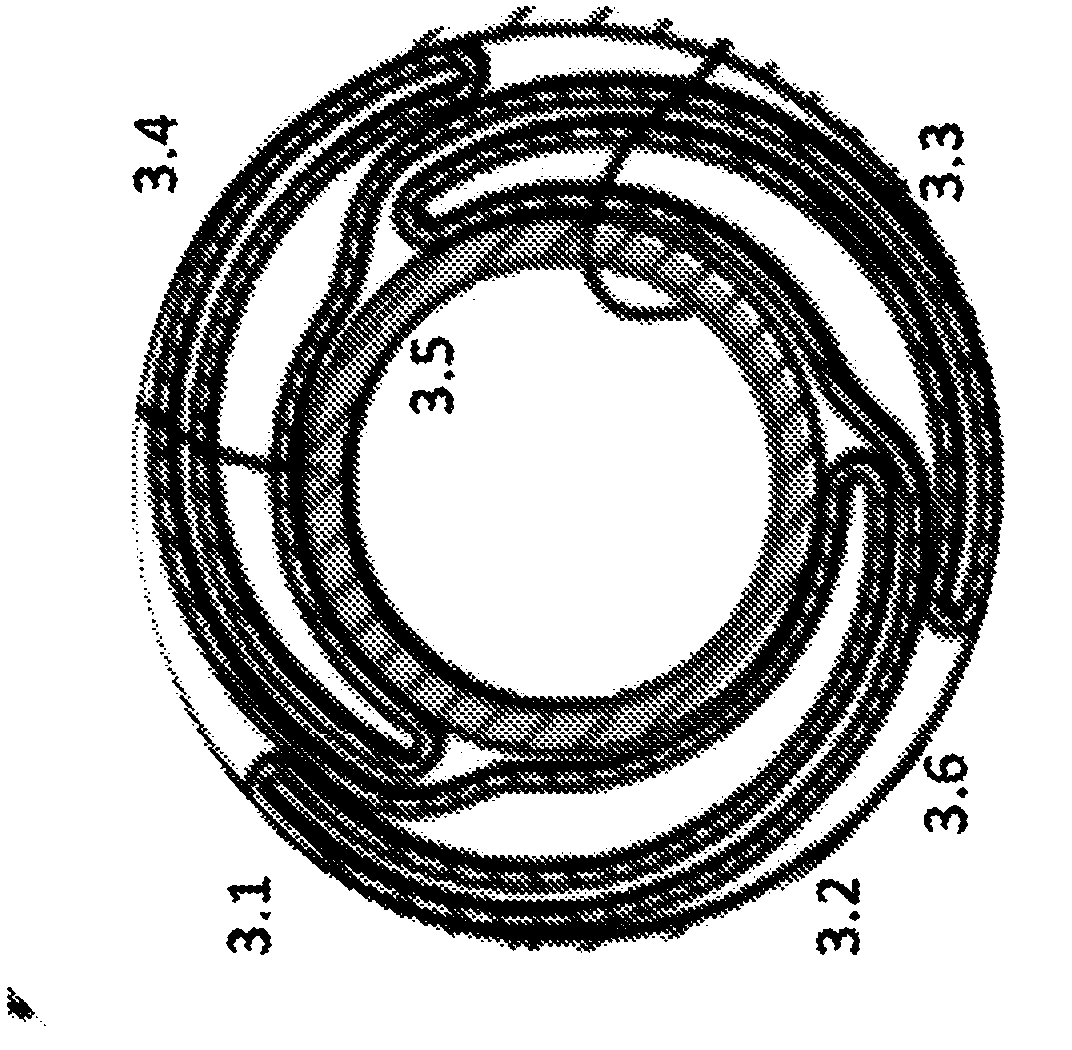
Drug eluting balloon
A technology of balloons and medicaments, applied in the direction of drug combination, drug delivery, drug devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: Balloon Coating (Paclitaxel)
[0118] (a) 90% paclitaxel / % Mpeg-PLGA in chloroform: Paclitaxel was mixed in chloroform to a w / v concentration of 1.5% (15 mg / mL). The polymer was mixed in chloroform to a w / v concentration of 1.5% (15 mg / mL). The two solutions were then mixed in a ratio of 90:10, respectively.
[0119] (b) 90% Paclitaxel / 10% Mpeg-PDLA in chloroform: Paclitaxel was mixed in chloroform to a w / v concentration of 1.5% (15 mg / mL). The polymer was mixed in chloroform to a w / v concentration of 1.5% (15 mg / mL). The two solutions were then mixed in a ratio of 90:10, respectively.
[0120] (c) 90% Paclitaxel / 10% Iohexol in distilled (DI (deionized water)) water: Paclitaxel was mixed in acetone to a w / v concentration of 1.5% (15 mg / mL). Iohexol was mixed in deionized water to a w / v concentration of 1.5% (15 mg / mL). The two solutions were then combined in a 90:10 or 95:5 ratio (if applicable).
[0121] (d) 90% Paclitaxel / 10% Urea in DI water: Paclita...
Embodiment 2
[0124] Example 2: Balloon Coating (Sirolimus)
[0125] (a) 90% Sirolimus / 10% Mpeg-PLGA in chloroform: Sirolimus was mixed in chloroform to a w / v concentration of 1.5% (15 mg / mL). The polymer was mixed in chloroform to a w / v concentration of 1.5% (15 mg / mL). The two solutions were then mixed in a ratio of 90:10, respectively.
[0126] (b) 90% sirolimus / 10% Mpeg-PDLA in chloroform: Sirolimus was mixed in chloroform to a w / v concentration of 1.5% (15 mg / mL). The polymer was mixed in chloroform to a w / v concentration of 1.5% (15 mg / mL). The two solutions were then mixed in a ratio of 90:10, respectively.
[0127] (c) 90% sirolimus / 10% iohexol in distilled (DI) water: Sirolimus was mixed in acetone to a w / v concentration of 1.5% (15 mg / mL). Iohexol was mixed in deionized water to a w / v concentration of 1.5% (15 mg / mL). The two solutions were then combined in a 90:10 or 95:5 ratio (if applicable).
[0128] (d) 90% sirolimus / 10% urea in DI water: Sirolimus was mixed in chlorofo...
Embodiment 3
[0131] Example 3: Elution Profile
[0132] (a) Kinetics of the elution profile: Coated balloons were stored in different 1 mL PBS aliquots at 37°C for a series of defined times with and without the expandable cover , such as 30 seconds, 1, 2, 3, 4, 5, 10, 15, 30, 60, and 120 minutes, to generate an elution profile over time. Aliquots of PBS were then analyzed by high pressure liquid chromatography (HPLC) to establish the concentration of sirolimus in solution at each time point. Calibration standards containing known amounts of sirolimus will be used to determine the amount of sirolimus eluted. The multiple peaks present for sirolimus (also present in the calibration standards) were summed to give the amount of sirolimus eluted over that time period (in absolute amounts and as eluted cumulative amounts). High pressure liquid chromatography (HPLC) analysis was then performed using a Waters HPLC system.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com