Preparation process of azilsartan micro-powder raw material drug
A technology of preparation process and raw material medicine, applied in the direction of organic chemistry, etc., can solve the problems of cumbersome crushing process operation, increased impurities, and difficulty in micronization of azilsartan raw material medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
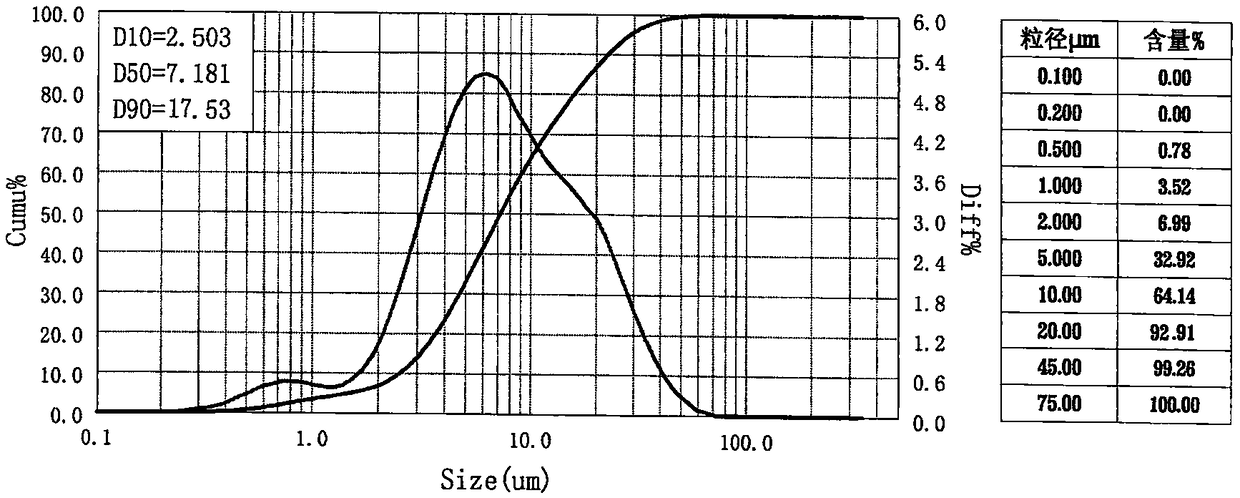
[0049] Get 100g of azilsartan bulk drug and place it in the RF-08 type high-speed pulverizer, pulverize intermittently for 2 minutes, and the HPLC and particle size of the gained azilsartan micropowder sample are shown in the following table:
[0050]
Embodiment 2
[0052] Get 100g azilsartan crude drug and place it in the RF-08 type high-speed pulverizer, pulverize intermittently for 5 minutes, and the obtained azilsartan micropowder sample HPLC and particle size are shown in the following table:
[0053]
[0054] B, the process described in the present invention
Embodiment 3
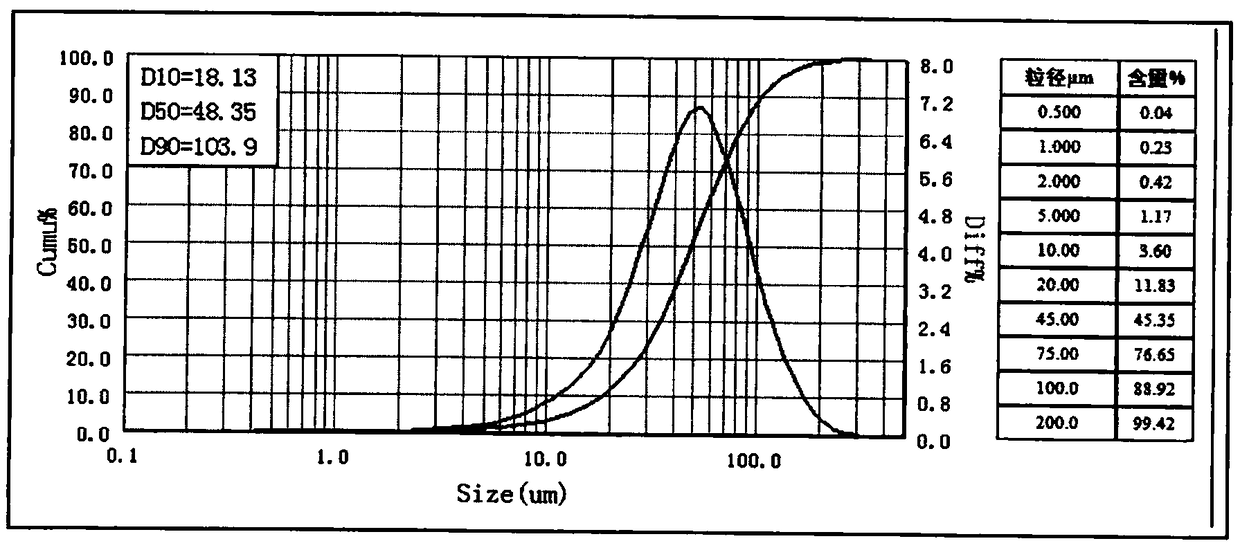
[0056] Put 25.0g of azilsartan raw material drug and 100ml of tetrahydrofuran into a reaction bottle, heat to 60-70°C and stir for 1 hour, then place the suspension to cool down to 20-30°C, filter, and wash the filter cake with 50ml of tetrahydrofuran to obtain The filter cake was dried under reduced pressure at 30-40° C. to obtain 26.1 g of dispersed azilsartan. Put the dispersed azilsartan and 1430ml of acetone into a 2L reaction bottle, heat to 55-65°C to dissolve, then distill 620ml of acetone, after the distillation, cool down to 25-30°C at a rate of 10°C / hour, and Stir at 25-30°C for 1 hour, then lower the temperature to 0-5°C, keep stirring at 0-5°C for 2 hours, filter, wash the filter cake with 50ml of cold acetone, and dry it in vacuum at 30-40°C to obtain Azisa Tan 23.7g, yield 94.8%. HPLC purity and particle size are shown in the table below:
[0057]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap