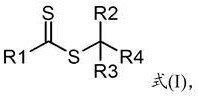
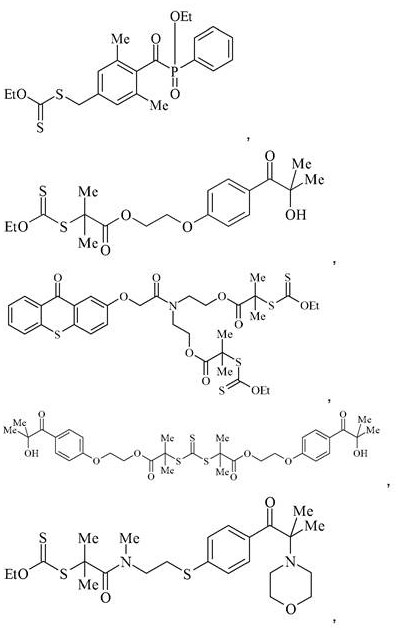
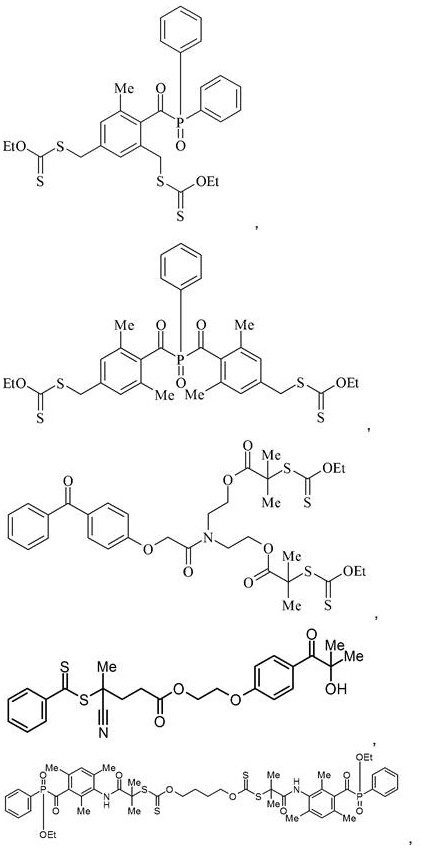
Photoinitiators and curable compositions
一种光引发剂、光引发的技术,应用在周期表第5/15族元素的化合物、有机化学、化学仪器和方法等方向,能够解决迁移和移动等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] THIOXANTHON-1 is a polymerizable thioxanthone with the following structure and is a 50% by weight solution in VEEA. THIOXANTHON-1 was prepared according to Example 1 of EP 2684876 A (AGFA):
[0268] .
[0269] GENOPOL AB1 is a polymeric 4-dimethylaminobenzoic acid derivative supplied by Rahn.
[0270] VEEA is 2-(vinylethoxy)ethyl acrylate available from NIPPON SHOKUBAI, Japan.
[0271] INHIB is a mixture of polymerization inhibitors with a composition according to Table 2:
[0272] Table 2
[0273]
[0274] Cupferron TM AL is aluminum N-nitrosophenylhydroxylamine from WAKO CHEMICALS LTD.
[0275] The PET100 substrate is a 100 μm thick transparent polyethylene terephthalate substrate available from AGFA-GEVAERT.
[0276] Measurement methods
[0277] 1. Molecular weight
[0278] Molecular weights of compounds were determined using TLC-MS according to the following procedure. TLC was performed as given in the synthetic examples. Using an Agilent 1100 HPLC p...
Embodiment 2
[0291] This example illustrates the reduced extractability of RAFT functionalized acylphosphine oxide INI-RAFT-1. Comparisons were made with the commercial photoinitiator TPO-L and the comparative RAFT functionalized photoinitiator COMP-RAFT-1.
[0292] Synthesis of COMP-RAFT-1 (step 2)
[0293]
[0294] Dissolve 3g (7.6mmol) (4-bromomethyl-2,6-dimethyl-benzoyl)-phenyl-phosphinic acid ethyl ester in 30ml ethanol, and add to 2.74g (12.2mmol) di In a solution of ethyl dithiocarbamate sodium salt and 0.113 g (0.75 mmol) sodium iodide in 40 ml ethanol. The reaction mixture was heated to 78°C for 4 hours. The mixture was cooled to room temperature. The solvent was removed under reduced pressure, and using preparative column chromatography on a Prochrom TM Purification of crude COMP-RAFT-1 on LC80 column using Kromasil TM C18 100 Å 10 µm as silica and methanol / 0.2M ammonium acetate as eluent. 0.98 gCOMP-RAFT-1 was isolated (in Reveleris provided by GRACE TM TLC analysis...
Embodiment 3
[0310] This example illustrates the synthesis of photoinitiators according to the invention.
[0311] Synthesis of INI-RAFT-2
[0312]
[0313] Step 1: Irgacure TM Acylation of 2959
[0314] 9g (40mmol) Irgacure TM 2959 was dissolved in 150ml ethyl acetate at 40°C. 4.45 g (44 mmol) triethylamine were added. A solution of 9.20 g (40 mmol) 2-bromo-2-methyl-propionyl bromide in 25 ml ethyl acetate was added dropwise and the reaction was continued at room temperature for 16 hours. A further 2.23 g (22 mmol) of triethylamine were added, followed by 4.6 g (20 mmol) of 2-bromo-2-methyl-propionyl bromide in 10 ml of ethyl acetate. The reaction was allowed to continue for another 1 hour at room temperature. Precipitated salts were removed by filtration. The ethyl acetate fraction was washed twice with a mixture of 50 ml water and 50 ml brine, washed over MgSO 4 Dry and evaporate under reduced pressure. The acylation product is in GraceResolve provided by GRACE TM Purific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fluorescence wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| viscosity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com