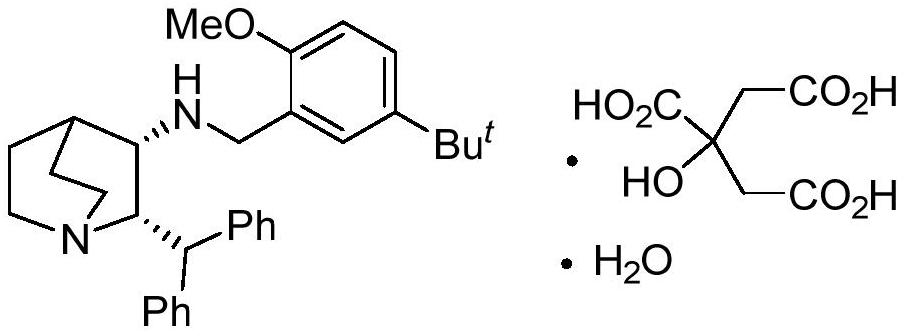
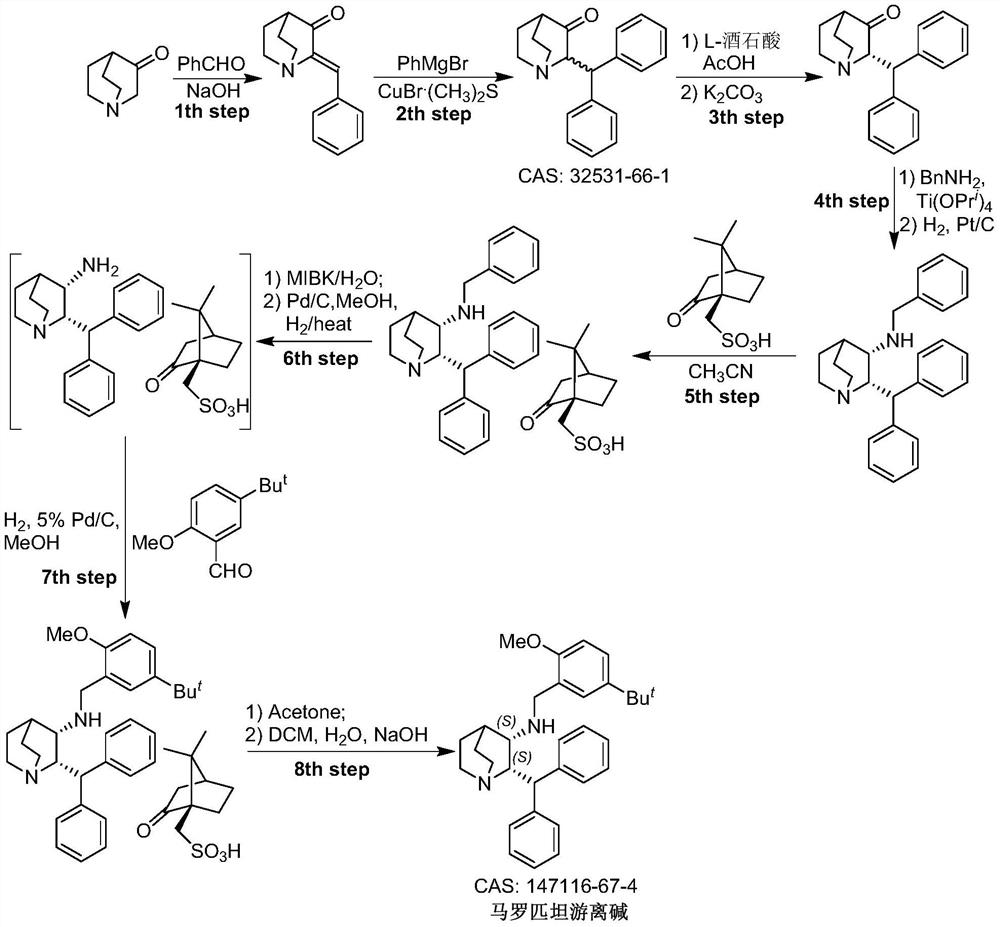
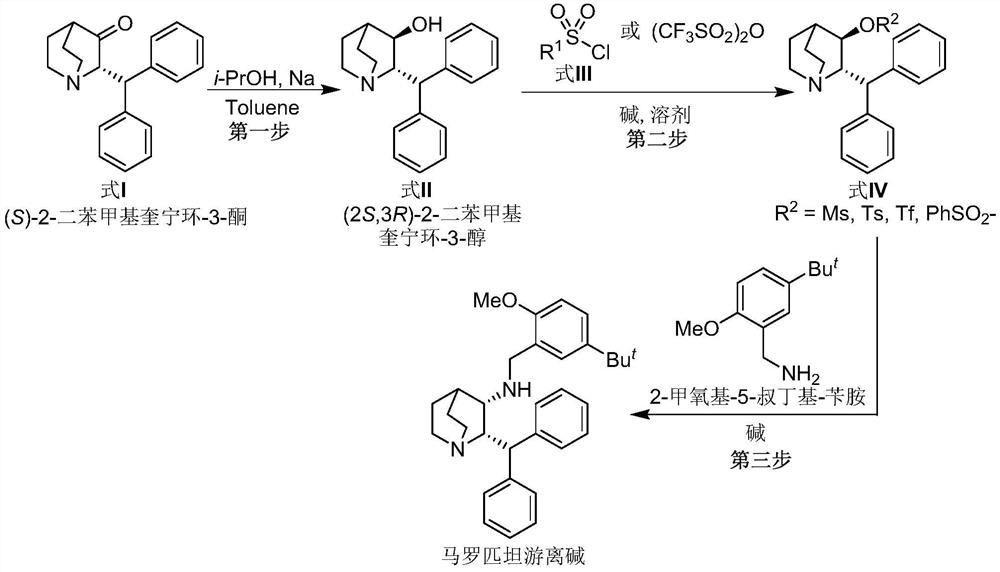
A kind of preparation method of maropitant free base
A technology of free base and reaction formula, which is applied in the field of preparation of raw material drug maropitant free base, can solve the problems of consuming a large amount of solvent and adsorbent, high cost, and long route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one: the preparation of (2S, 3R)-2-benzhydrylquinuclidin-3-alcohol (formula II)
[0024] Add (S)-2-benzhydrylquinuclidin-3-one (Formula I) (130g, 446mmol, 1.0eq.) and toluene (1.8L) into a 5L reaction flask, and heat the system under reflux to separate water after the addition , to remove a small amount of water in the reaction system. Then metal Na (50 g, 2175 mmol) cut into small pieces was slowly added under reflux, and i-PrOH (450 mL) was slowly added under reflux after the addition was complete. After the system was refluxed for 1.5 hours, the temperature was naturally cooled to room temperature, and methanol (1 L) was added to the reaction system to quench the reaction. The system was concentrated under high vacuum and reduced pressure to remove the solvent, and the residue was added to H 2 O(2L) and CH 2 Cl 2 (1L), the system was stirred for 3 hours and left to stand, the organic phase was separated, and the aqueous phase was CH 2 Cl 2 (3 × 500mL...
Embodiment 2
[0025] Embodiment two: (2S, 3R)-2-benzhydryl-3-methanesulfonyloxy-quinine (formula IV, R 2 = Preparation of Ms)
[0026] Add (2S,3R)-2-benzhydrylquinuclidin-3-ol (Formula II) (5.0 g, 17 mmol) and CH to the reaction flask 2 Cl 2 (15 mL). The reaction system was cooled to 5°C in an ice-salt bath, and then Pyridine (25 mL) and MsCl (4.0 g, 34.9 mmol) were slowly added through the dropping funnel. After the addition, the system was naturally warmed to room temperature and stirred for 5 hours, then heated to reflux for 2 hours, and then the system was naturally cooled to room temperature. Add HCl solution (2N in H 2 O) adjust the pH value of the system to 8-9, then add CHCl 3 (3×120mL) was extracted three times, the organic phases were combined, the organic phase was concentrated under high vacuum to remove the solvent, and the residue was purified by column chromatography to obtain (2S,3R)-2-benzhydryl-3-methanesulfonyloxy- Quinine (formula IV, R 2 =Ms) (5.15 g, 81.5%).
Embodiment 3
[0027] Embodiment three: the preparation of maropitant free base
[0028] Add (2S, 3R)-2-benzhydryl-3-methanesulfonyloxy-quinine (formula IV, R) in the reaction flask 2 =Ms) (4.5 g, 12.11 mol) and DMF (20 mL). After the addition was complete, triethylamine (4.9 g, 48.42 mmol, 4.0 eq.) and 2-methoxy-5-tert-butyl-benzylamine (3.50 g, 18.11 mol) were added to the system. After the addition, the temperature of the system was raised to 100°C to react until the disappearance of the starting material was tracked by TLC. The system was naturally cooled to room temperature, the organic solvent was removed under high vacuum, and the residue was added with CH 2 Cl 2 (60mL) and H 2 O (60mL), the organic phase was separated, and the aqueous phase was separated with CH 2 Cl 2 Extract (3 x 30 mL). The organic phases were combined, and the organic phase was washed with saturated brine (50mL), anhydrous Na 2 SO 4 After drying, the solvent was precipitated under reduced pressure, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com