A kind of salidroside derivative and its preparation method and application
A technology of salidroside and compounds, applied in the field of medicine, can solve the problems of reducing drug dosage, strong hydrophilicity, and reducing drug efficacy, and achieve the effect of overcoming low bioavailability and good apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
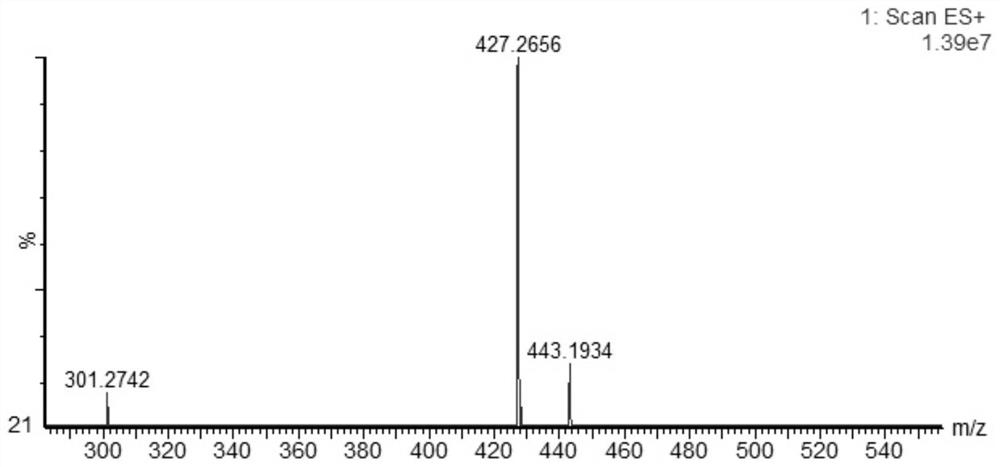
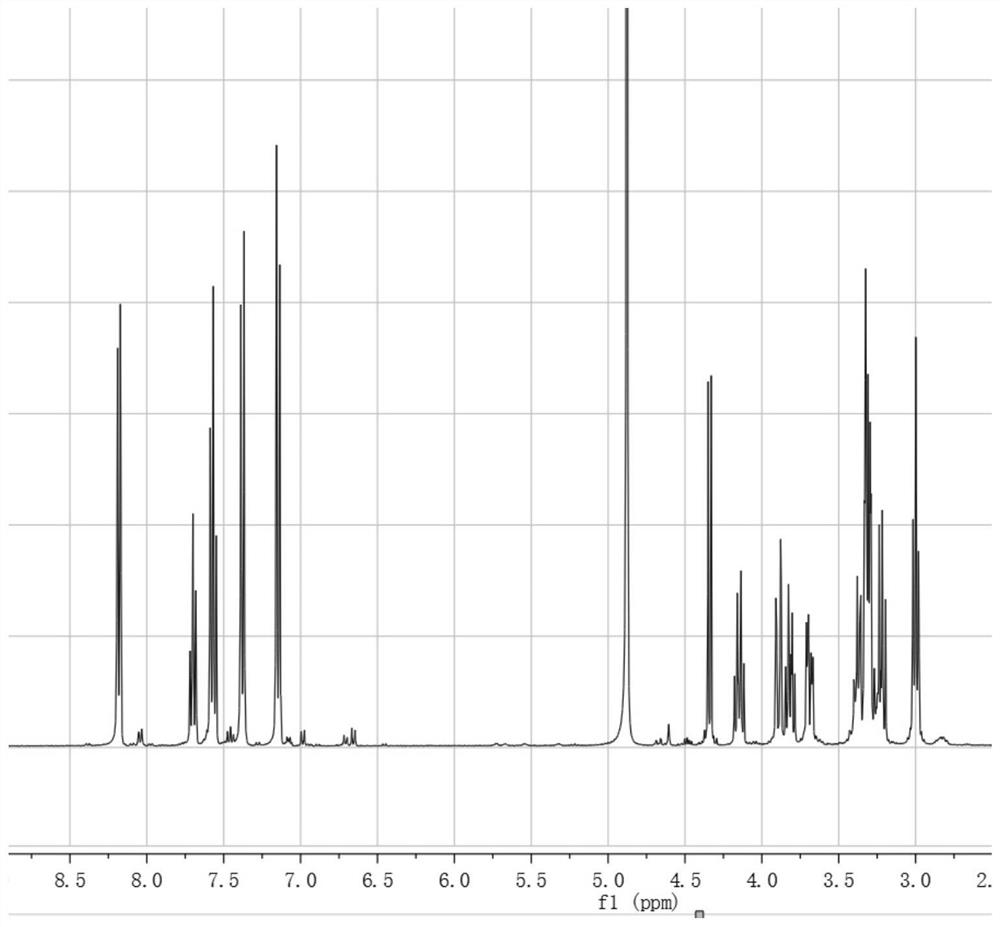
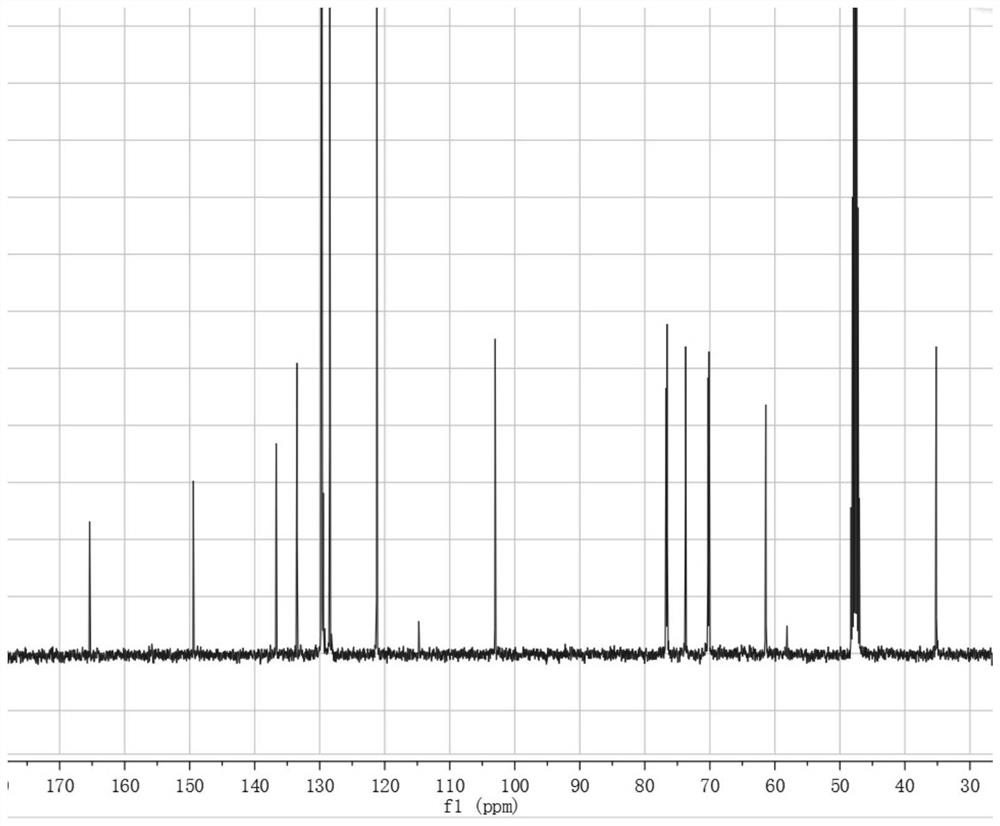
[0033] The preparation of embodiment 1 salidroside derivatives
[0034] Add 3g (0.01mol) salidroside, 1.06g (0.01mol) sodium carbonate, 0.05g tetrabutylammonium bromide, and 10ml water into a 50ml three-necked flask. In an ice bath, stir until the solid is completely dissolved and the temperature of the system drops below 5°C. Dissolve 1.3ml (0.01mol) of benzoyl chloride in 10ml of ethyl acetate and add to the system. TLC (CHCl 3 :CH 3 OH=4:1) monitor the reaction, and stop the reaction when the raw material point disappears. The liquid was separated, and the aqueous phase was extracted three times with ethyl acetate, each time using 10 ml of ethyl acetate. The organic phases were combined, dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a white solid. With silica gel as the stationary phase, (CHCl 3 -CH 3 OH) as the eluent, gradient elution was performed using flash preparative chromatography (model: Biotage isolera ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com