A Class of Fluorescent Probes for Multicolor Imaging of Lipid Droplet Nuclei with High Brightness
A fluorescent probe, high-brightness technology, applied in the field of fluorescence imaging, can solve the problems of large Stokes shift, signal overlap, increased experimental cost, etc., achieve high brightness and photostability, good rigidity and planarity, synthetic raw materials The effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of the probe OMor-Aze.
[0035] The intermediate Naph-Aze synthesis route and product structure are as follows:
[0036]
[0037] Weigh 4-bromo-1,8-naphthalene anhydride (3.0g, 10.9mmol), anhydrous copper sulfate (5.2g, 32.7mmol) in 10mL dry DMF, add azetidine (1.9mmol) under nitrogen protection g, 32.7 mmol), the temperature was raised to 140° C. to react for 20 h, and after cooling the reaction solution to room temperature, the reaction solution was slowly poured into ice water, and a large amount of orange solids were precipitated. The filtrate was removed by suction filtration, and the filter residue was dissolved in dichloromethane and purified by silica gel column chromatography (petroleum ether / dichloromethane=1 / 10, V / V) to obtain 1.1 g of orange solid powder with a yield of 40%.
[0038] Its high-resolution mass spectrometry data are as follows:
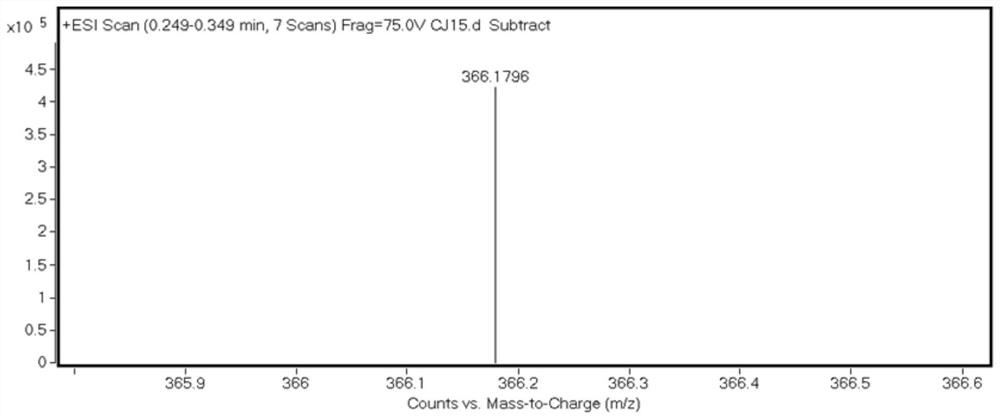
[0039] HRMS (ESI): m / z: [M+H] + : Calculated: 254.0817, Experimented: 254.0883.
[0040] After dete...
Embodiment 2
[0055] The synthesis route and product structure of the probe Mor-Aze are as follows:
[0056]
[0057] Weigh Naph-Aze (0.1g, 0.4mmol) in 5mL of absolute ethanol, add 4-(2-aminoethyl)morpholine (0.15g, 1.2mmol), heat the reaction solution to 80°C for 12h, The reaction solution was cooled to room temperature, and the solvent was distilled off under reduced pressure. The residue was separated through a silica gel column (dichloromethane / methanol=50 / 1, V / V) to obtain the product, which was 0.12 g of an orange solid, with a yield of 87%.
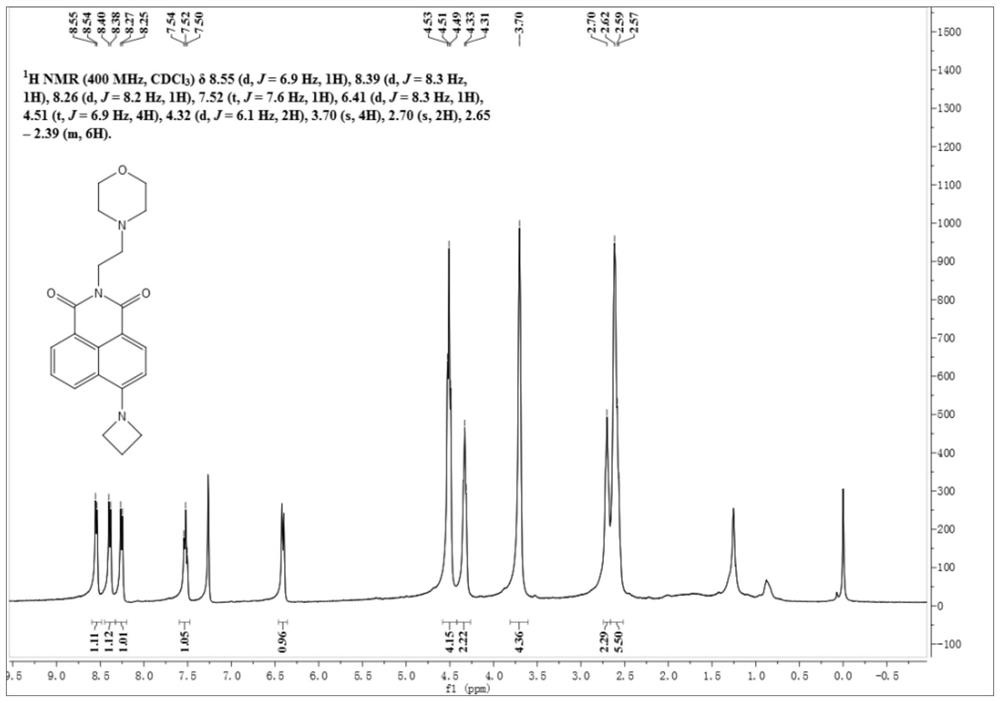
[0058] Its H NMR spectrum is as follows figure 1 As shown, the specific data are as follows:
[0059] 1 H NMR (400MHz, CDCl 3 , ppm): δ=8.55(d, J=6.9Hz, 1H), 8.39(d, J=8.3 Hz, 1H), 8.26(d, J=8.2Hz, 1H), 7.52(t, J=7.6Hz ,1H),6.41(d,J=8.3Hz,1H), 4.51(t,J=6.9Hz,4H),4.32(d,J=6.1Hz,2H),3.70(s,4H),2.70(s ,2H),2.65-2.39 (m,6H).
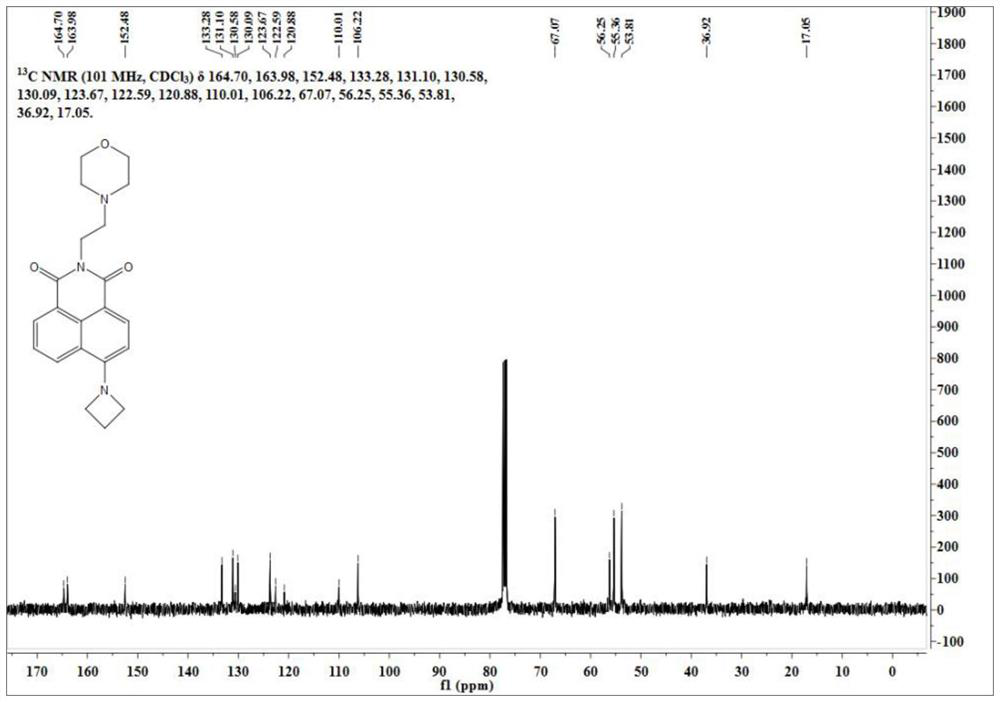
[0060] Its carbon NMR spectrum is as follows figure 2 As shown, the specific data are as follows:
[0061] 13 C...
Embodiment 3
[0069] The synthesis route and product structure of the probe DEma-Aze are as follows:
[0070]
[0071] Weigh Naph-Aze (0.1g, 0.4mmol) in 5mL of absolute ethanol, add N,N-dimethylethylamine (0.1g, 1.2mmol), heat the reaction solution to 80°C for 10h, the reaction solution After cooling to room temperature, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (dichloromethane / methanol=50 / 1, V / V) to obtain 0.11 g of an orange solid with a yield of 86%.
[0072] Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0073] 1 H NMR (400MHz, CDCl 3 )δ8.51(dt, J=29.2, 14.7Hz, 1H), 8.40-8.29(m, 1H), 8.24-8.14(m, 1H), 7.49(td, J=8.5, 2.3Hz, 1H), 6.44 -6.29(m,1H),4.61-4.41(m,4H),4.32(t,J=7.2Hz,2H),2.66(t,J=7.2Hz,2H),2.61-2.52(m,2H), 2.37(s, 6H).
[0074] Its nuclear magnetic spectrum carbon spectrum data are as follows:
[0075] 13 C NMR (101MHz, CDCl 3 )δ164.77, 164.06, 152.51, 133.34, 131.16, 130.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com