Synthesizing method of metal cyclo-N5-salt
A metal salt and ring technology, applied in the field of compounds, can solve problems that hinder the application of high energy density materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] The present invention will be described in detail below in combination with specific embodiments.
[0016] For the most stable structure of a compound at a given pressure, the enthalpy of formation per atom is calculated using the following formula:
[0017] h f (CuN x )=[H(CuN x )-H(CuN 3 )-(x-3)H(N 2 ) / 2] / (x+1)
[0018] where H f is the enthalpy of formation per atom, H is the calculated enthalpy of one chemical unit per compound, CuN x and CuN 3 The enthalpy H is calculated by the CALYPSO method to search for the lowest energy structure at the set pressure. for N 2 , known structures with Pa-3 symmetry are considered to have a lower enthalpy of formation in the molecular nitrogen phase in the pressure range from 0 to 100 GPa.
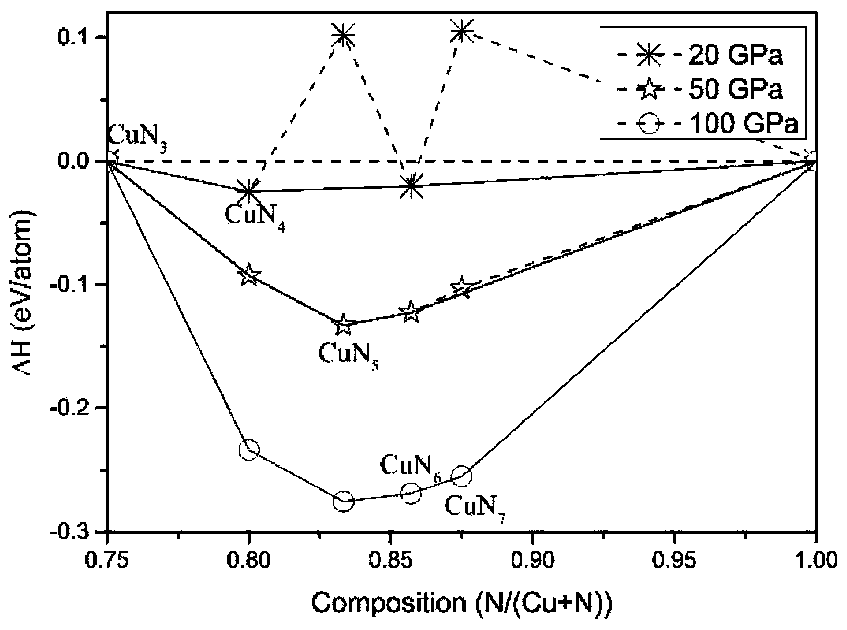
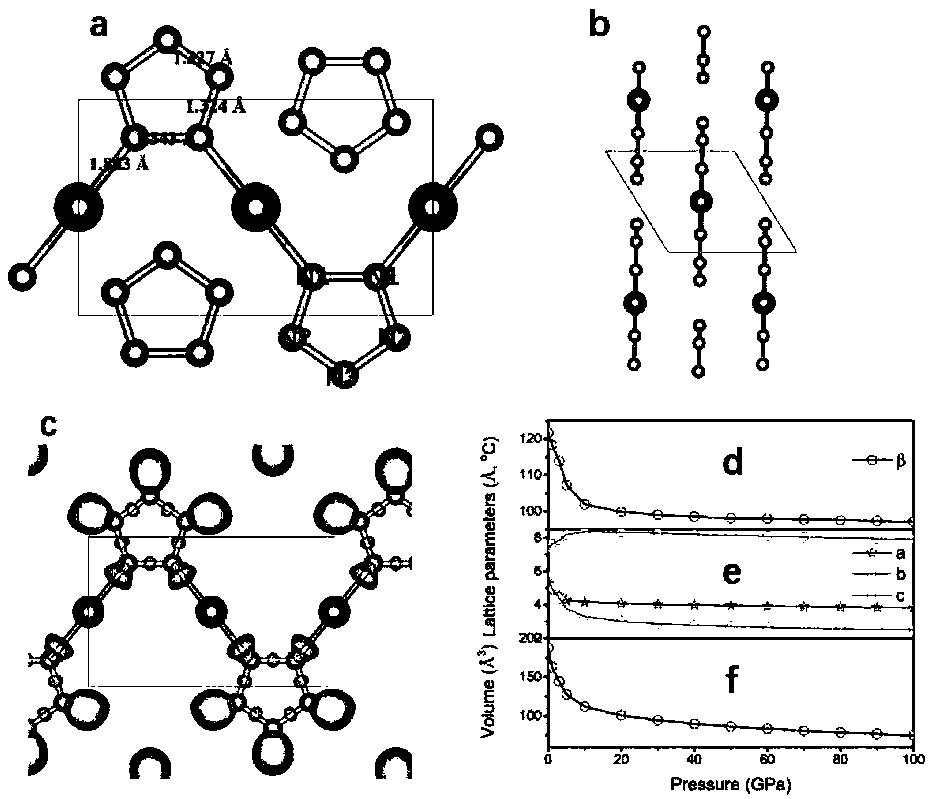
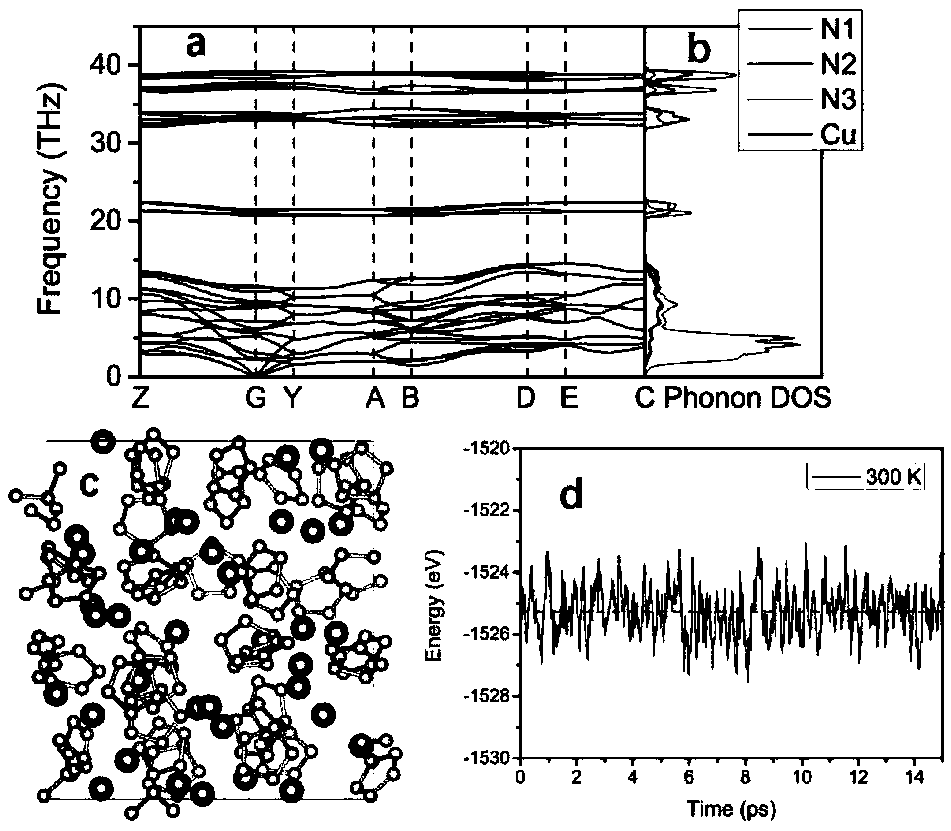
[0019] The present invention studies CuN x The energy stability of the system at high pressure was calculated for various CuN x The enthalpy of formation of a compound in the pressure range from 0 to 100 GPa, as figure 1shown. Cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com