Genetic engineered T cell and application
A genetic engineering and cell technology, applied in the field of T lymphocytes, specifically inducing the expression of cytokines, can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1I
[0212] Example 1. Preparation of IL12-GPC3-CAR T cells
[0213] 1. Plasmid construction
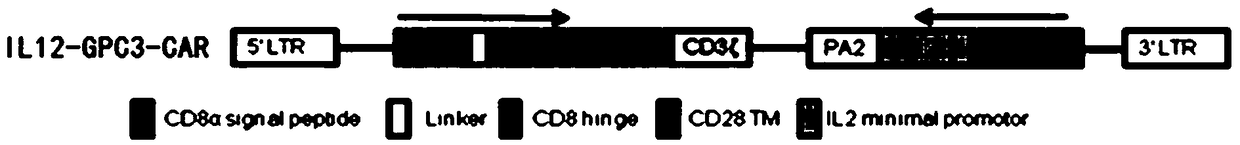
[0214] The scFv used in this example is an antibody targeting GPC3, and its nucleic acid sequence is shown in SEQ ID NO: 1. The chimeric antigen receptor used is a second-generation chimeric antigen receptor, which has a CD28 transmembrane domain, a CD28 intracellular domain, and CD3ζ. refer to figure 1 As shown in the plasmid map, the plasmid for IL12-GPC3-CAR T cells was constructed.
[0215] Using pRRLSIN.cPPT.EF-1α as the vector, a lentiviral plasmid pRRLSIN.cPPT.EF-1α-GPC3-CAR-T expressing the second-generation chimeric antigen receptor was constructed. The GPC3-CAR-T sequence consists of CD8α signal peptide (SEQ ID NO: 2), scFv targeting GPC3 (SEQ ID NO: 1), CD8hinge (SEQ ID NO: 3), CD28 transmembrane region (SEQ ID NO: 6 ) and the intracellular signaling domain (SEQ ID NO: 4) and the intracellular segment of CD3 CD3ζ (SEQ ID NO: 5).
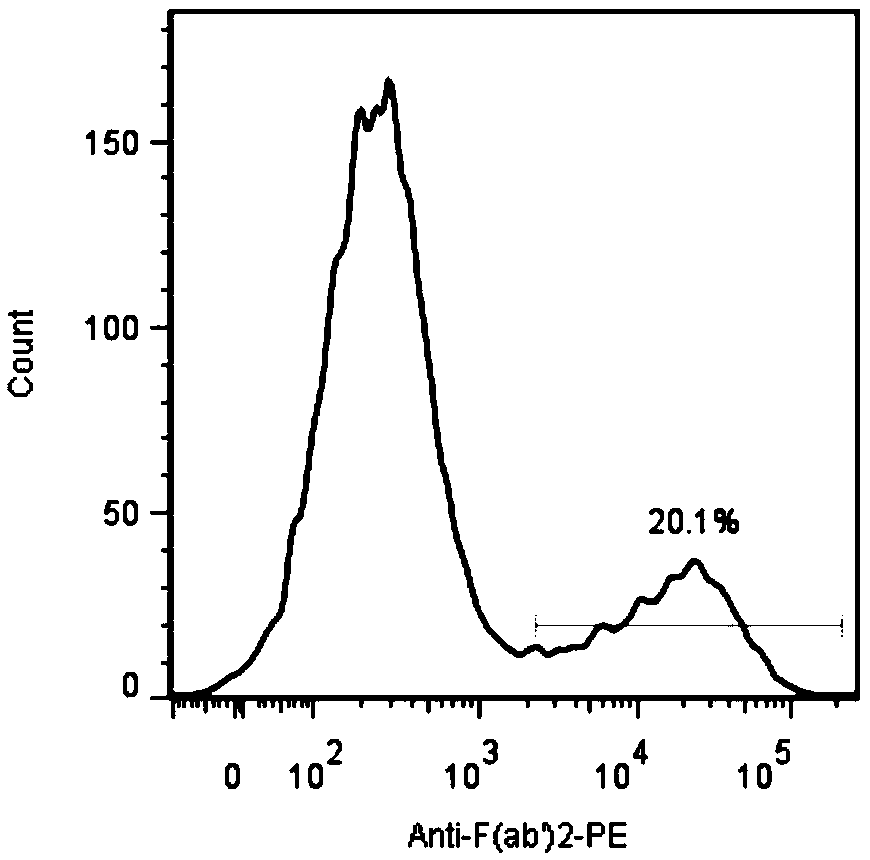
[0216] IL12-GPC3-CAR T cells were inserted...
Embodiment 2
[0240] Example 2. Construction of TCR-deficient IL12-GPC3-CAR T cells
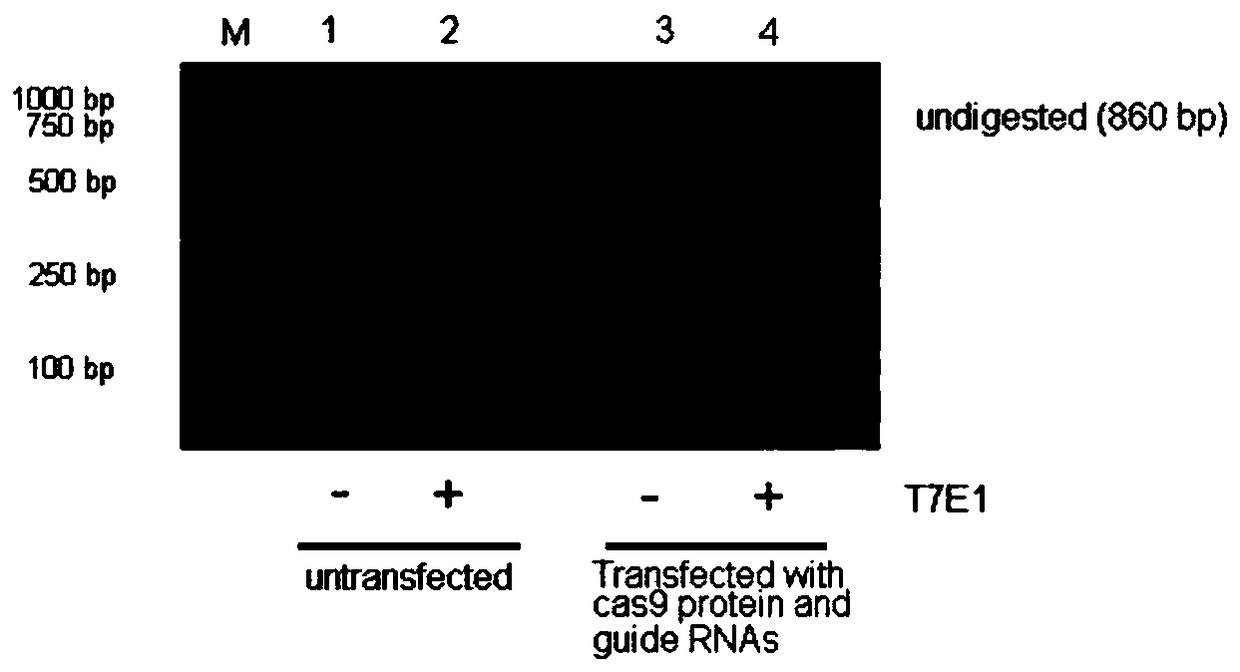
[0241] 1. Construction of CRISPR and in vitro transcription of corresponding gRNA
[0242] The two gRNAs used to knock out the TCRα chain are targeting the first exon of the constant region of the TCRα chain. The two gRNA target sequences are:
[0243] TRAC-gRNA-1 (SEQ ID NO: 13):GAGTCTCTCAGCTGGTACA
[0244] TRAC-gRNA-2 (SEQ ID NO: 14): TGTGCTAGACATGAGGTCTA.
[0245] The gRNA in vitro transcription box driven by the T7 promoter is as follows: T7Promoter+target gRNA+guide RNAScaffold
[0246] TRAC-gRNA-1-IVT-Template (SEQ ID NO: 15):
[0247] TAATACGACTCACTATAGAGAGTCTCTCAGCTGGTACAGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTTTTT
[0248] TRAC-gRNA-2-IVT-Template (SEQ ID NO: 16):
[0249] TAATACGACTCACTATAGTGTGCTAGACATGAGGTCTAGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTTTTT
[0250] After the DNA sequence of the above TRAC-gRNA-1 / 2-I...
Embodiment 3
[0262] Example 3. Cytotoxicity assay of IL12-GPC3-CAR T cells before and after TCR inactivation
[0263] The CytoTox 96 non-radioactive cytotoxicity detection kit (Promega Company) was used to detect the cytotoxicity. For details, refer to the instructions of the CytoTox 96 non-radioactive cytotoxicity detection kit.
[0264] 1) Target cells: Inoculate 50 μL 2×10 5 / mL of Huh-7, PLC / PRF / 5, SK-HEP-1 cells in 96well plate, wherein, Huh-7 cells, PLC / PRF / 5 cells are GPC3 positive, SK-HEP-1 cells are GPC3 negative;
[0265] 2) Effector cells: IL12-GPC3-CAR T cells added with TCR+ or TCR- according to the effect-to-target ratio of 1:1;
[0266] 3) There are 5 replicate wells in each group, and the average value of the 5 replicate wells is taken. The detection time is the 18th hour. Wherein each experimental group and each control group are as follows:
[0267] Each experimental group: each target cell + the above-mentioned effector cells; control group 1: target cells release LD...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap