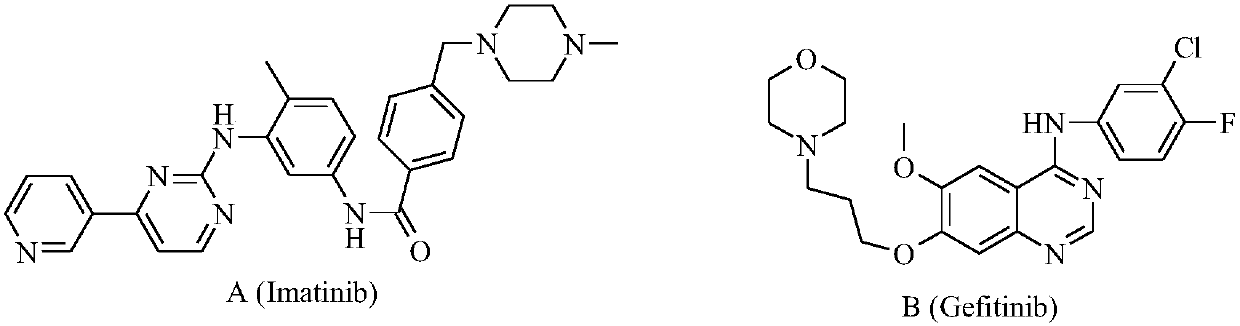
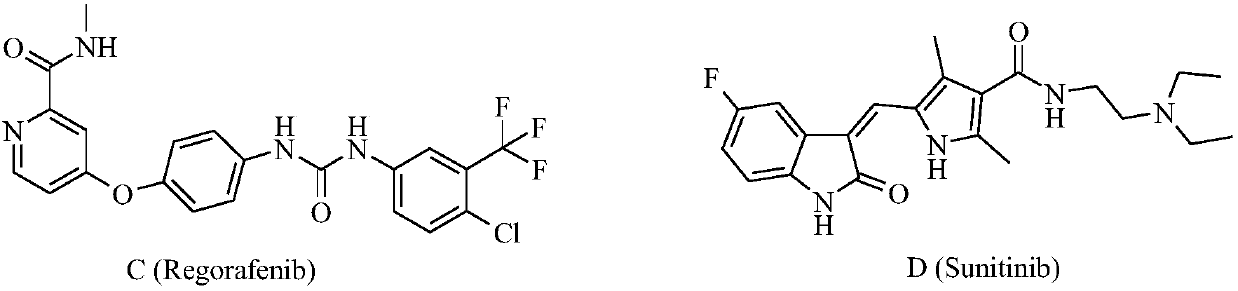
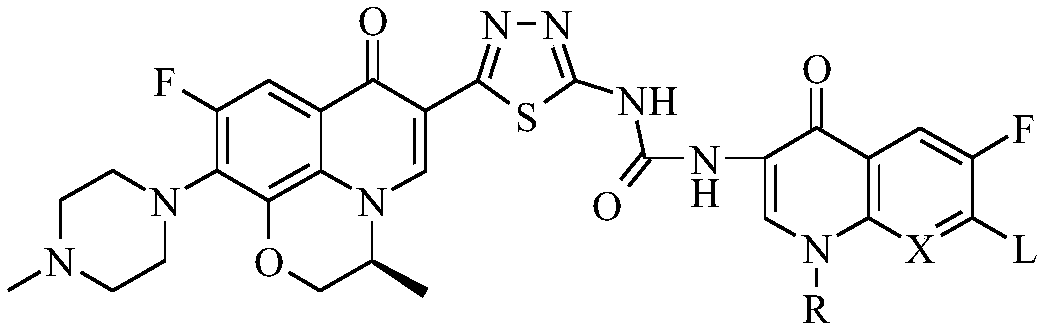
Preparation and application of bis-fluoroquinolone thiadiazole urea levofloxacin derivative
A technology of fluoroquinolone thiadizuron and levofloxacin, applied to antineoplastic drugs, the field of bis-fluoroquinolone thiadizuron levofloxacin derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (S)-1-{2-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)-quinoline-4(1H) -Keto-3-yl]-1,3,4-thiadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-( 1,3-oxypropyl)-quinoline-4(1H)-one-3-yl]-urea (I-1), its chemical structural formula is:
[0039]
[0040] The preparation method of the bis-fluoroquinolone thiadizuron of this embodiment is: take levofloxacin hydroxamic acid (1″) 1.0g (2.7mmol) and suspend in 25mL acetonitrile, add carbonyldiimidazole (CDI) 0.50g (3.2mmol ), stirring at room temperature until the material dissolves. Then add 1.10 g (2.7 mmol) of levofloxacin C-3 thiadiazole amine IV intermediate, and stir in a water bath at 55 to 60 ° C for 12 hours. Place overnight, collect the solid produced by filtration, and wash with acetonitrile. Crude product Recrystallized with DMF-ethanol mixed solvent to obtain pale yellow crystal (I-1), with a yield of 52%, m.p.224-226°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 11.56 (brs, 1H, NH), 9.44 (s, 1H, NH), 9.1...
Embodiment 2
[0042] (S, S)-1-{2-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)-quinoline-4( 1H)-keto-3-yl]-1,3,4-thiadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1 -(1,3-oxopropyl)-quinoline-4(1H)-one-3-yl]-urea (I-2), its chemical structural formula is:
[0043]
[0044] The preparation method of the bis-fluoroquinolone thiadizuron of this embodiment is: take levofloxacin hydroxamic acid (2″) 1.0g (2.7mmol) and suspend in 25mL acetonitrile, add carbonyldiimidazole (CDI) 0.60g (3.7mmol ), stirring at room temperature until the material is dissolved. Then add 1.1 g (2.7 mmol) of levofloxacin C-3 thiadiazole amine IV intermediate, and stir in a water bath at 55 to 60° C. for 16 hours. Place overnight, collect the solid produced by filtration, and wash with acetonitrile. Crude product Recrystallized with ethanol to obtain a light yellow crystal (I-2), with a yield of 46%, m.p.212-214°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 11.57 (brs, 1H, NH), 9.45 (s, 1H, NH), 9.16, 9.14 (2...
Embodiment 3
[0046] (S)-1-{2-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)-quinoline-4(1H) -Keto-3-yl]-1,3,4-thiadiazol-5-yl}-3-[6,7-difluoro-8,1-(1,3-oxopropyl)-quinoline- 4(1H)-ketone-3-yl]-urea (I-3), its chemical structural formula is:
[0047]
[0048] The preparation method of the bis-fluoroquinolone thiadizuron of the present embodiment is: take 1.0 g (3.4 mmol) of oxyfluorocarboxylic acid hydroxamic acid (II-3″) and suspend it in 25 mL of acetonitrile, add carbonyldiimidazole (CDI) 0.82g (5.1mmol), stirring at room temperature until the material is dissolved. Then add 1.40g (3.4mmol) of levofloxacin C-3 thiadiazole amine IV intermediate, and stir in a water bath at 55-60°C for 18 hours. Leave it overnight and collect the resulting The solid was washed with acetonitrile. The crude product was recrystallized from a mixed solvent of DMF-ethanol to obtain a light yellow crystal (I-3), with a yield of 57%, m.p.225-227°C. 1 H NMR (400MHz, DMSO-d 6 )δ:11.51(brs,1H,NH),9.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com