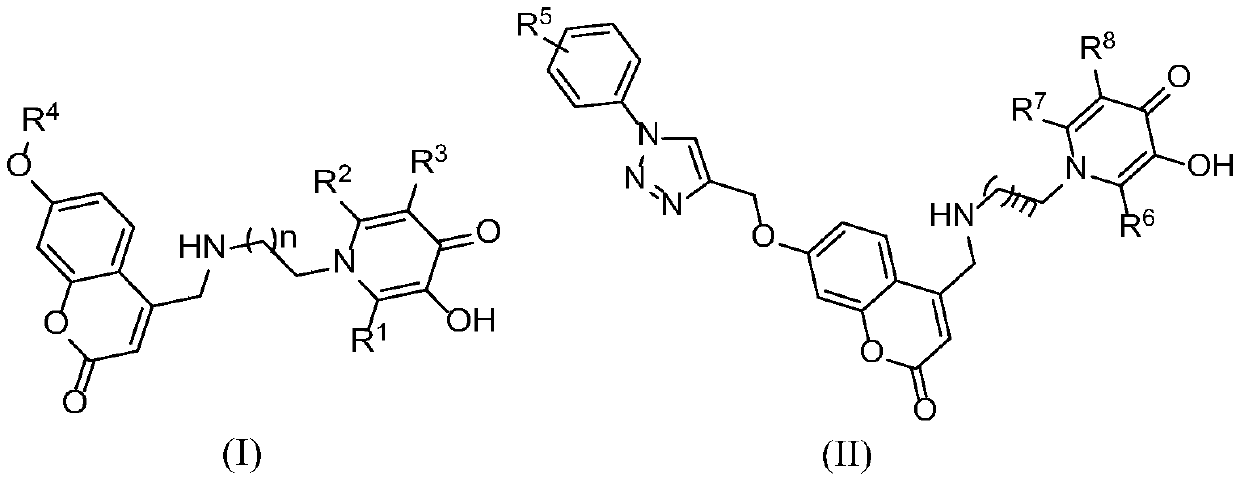
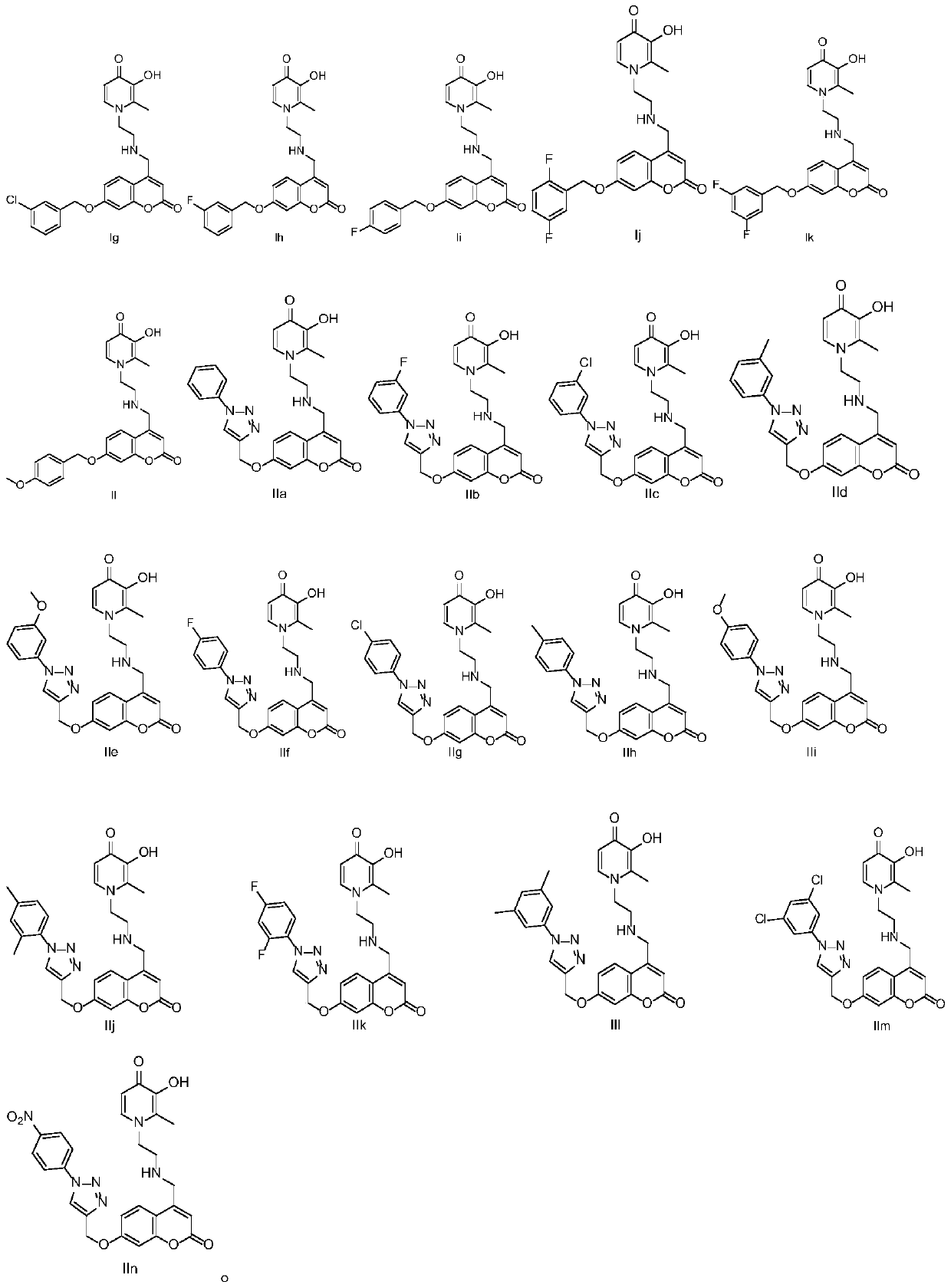
Derivative of coumarin parallel 3-hydroxypyridine-4-ketone and preparation method and application thereof
A hydroxypyridine and coumarin technology, which is applied in the field of coumarin and hydroxypyridone compounds for the treatment of Alzheimer's disease, can solve problems such as incomplete cure of the disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
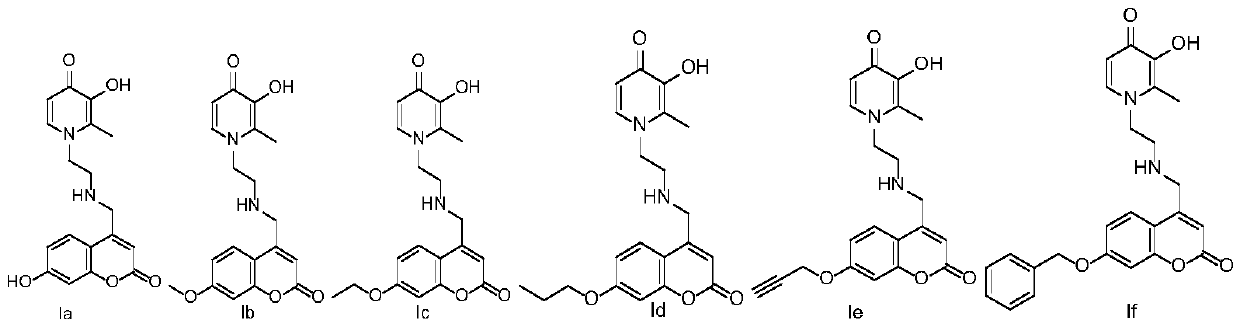
[0066] 3-hydroxy-1-(2-(((7-hydroxy-2-oxo-2H-benzopyran-4-yl)methyl)amino)ethyl)-2-methylpyridine-4(1H )-Ketone (Ia)
[0067] Add (3.303g, 30mmol) and 30mL of concentrated sulfuric acid to a 100mL single-necked flask, place it at -5°C and stir until it is completely dissolved, slowly add dropwise (6.600g, 40mmol) to control the reaction temperature and stir for 24h. The reaction system was poured into 300 mL ice water and stirred for 2 h, filtered and dried to obtain a white solid. The yield was 83%.
[0068] Add maltol (1.260g, 10mmol), potassium carbonate (2.740g, 20mmol), benzyl bromide (2.052g, 12mmol), 130mL acetone, and react at 56℃ for 2h in a 250mL single-necked flask, spin dry, add 100mL water to dissolve it, 80 mL of dichloromethane was extracted four times and the organic phase was combined by spin-drying to obtain a yellow oily liquid. The yield was 97%.
[0069] Take the product obtained in the second step (1.080g, 5mmol) in a 100mL single-necked flask, add 30mL ethan...
example 2
[0075] 3-hydroxy-1-(2-(((7-methoxy-2-oxo-2H-benzopyran-4-yl)methyl)amino)ethyl)-2-methylpyridine-4 (1H)-ketone (Ib)
[0076] Add (3.660g, 30mmol) and 30mL of concentrated sulfuric acid to a 100mL single-necked flask, place it at -5°C and stir until it is fully dissolved, and slowly add dropwise (6.600g, 40mmol) to control the reaction temperature and stir for 24h. The reaction system was poured into 300 mL ice water and stirred for 2 h, filtered and dried to obtain a white solid. The yield is 60%.
[0077] Add maltol (1.260g, 10mmol), potassium carbonate (2.740g, 20mmol), benzyl bromide (2.052g, 12mmol), 130mL acetone, and react at 56℃ for 2h in a 250mL single-necked flask, spin dry, add 100mL water to dissolve it, 80 mL of dichloromethane was extracted four times and the organic phase was combined by spin-drying to obtain a yellow oily liquid. The yield was 97%.
[0078] Take the product obtained in the second step (1.080g, 5mmol) in a 100mL single-necked flask, add 30mL ethanol...
example 3
[0084] 3-hydroxy-1-(2-(((7-ethoxy=yl-2-oxo-2H-benzopyran-4-yl)methyl)amino)ethyl)-2-methylpyridine -4(1H)-ketone (Ic)
[0085] Add (3.303g, 30mmol) and 30mL of concentrated sulfuric acid to a 100mL single-necked flask, place it at -5°C and stir until it is completely dissolved, slowly add dropwise (6.600g, 40mmol) to control the reaction temperature and stir for 24h. The reaction system was poured into 300 mL ice water and stirred for 2 h, filtered and dried to obtain a white solid. The yield was 83%.
[0086] Add the white solid (1.050g, 5mmol) obtained in the first step to 100mL, bromoethane (0.654g, 6mmol), potassium carbonate (0.959g, 7mmol), 40mL acetone, reflux for 4h, spin dry, add 40mL water, It was extracted 3 times with 40 mL of dichloromethane, combined and spin-dried to obtain a white yellow solid. The yield was 90%.
[0087] Add maltol (1.260g, 10mmol), potassium carbonate (2.740g, 20mmol), benzyl bromide (2.052g, 12mmol), 130mL acetone, and react at 56℃ for 2h in a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com