A kind of antineoplastic drug or drug carrier comprising cyclodextrin modified by glutamine
An anti-tumor drug, glutamine technology, applied in the field of anti-tumor drugs, can solve the problems of large batch-to-batch variation, difficulty in evaluating the safety of nano-carriers, limiting nano-carriers, etc., and achieve the effect of avoiding toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
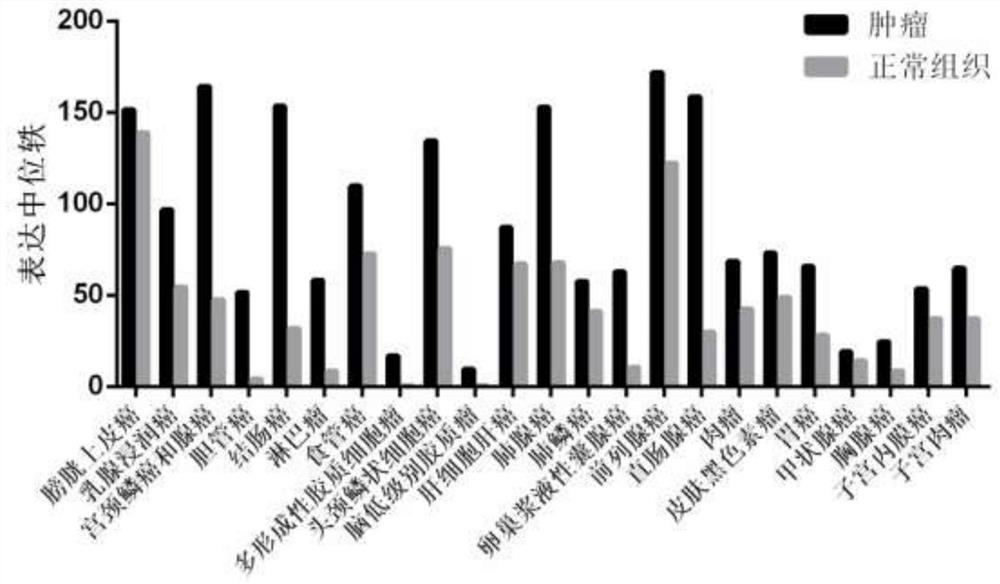
[0058] Example 1: Expression of glutamine transporter ASCT2 in various tumors.
[0059] The expression of ASCT2 in each tumor was obtained by querying the public database GEPIA, the search condition was "SLC1A5" (ASCT2 gene name), the sample size of each tumor was shown in Table 1, and the expression of ASCT2 in each tumor was as follows figure 1 Shown in bladder cancer, breast cancer, lung cancer, gastric cancer, hepatocellular carcinoma, melanoma, glioma, lymphoma, colorectal cancer, cervical cancer, bladder cancer, cholangiocarcinoma, esophageal cancer, head and neck squamous cell carcinoma, In ovarian cancer, prostate cancer, sarcoma, thyroid cancer, thymus cancer, endometrioma, sarcoma and other tumors, the expression of ASCT2 in tumor tissues is much higher than that in normal tissues.
[0060] Table 1
[0061]
[0062]
Embodiment 2
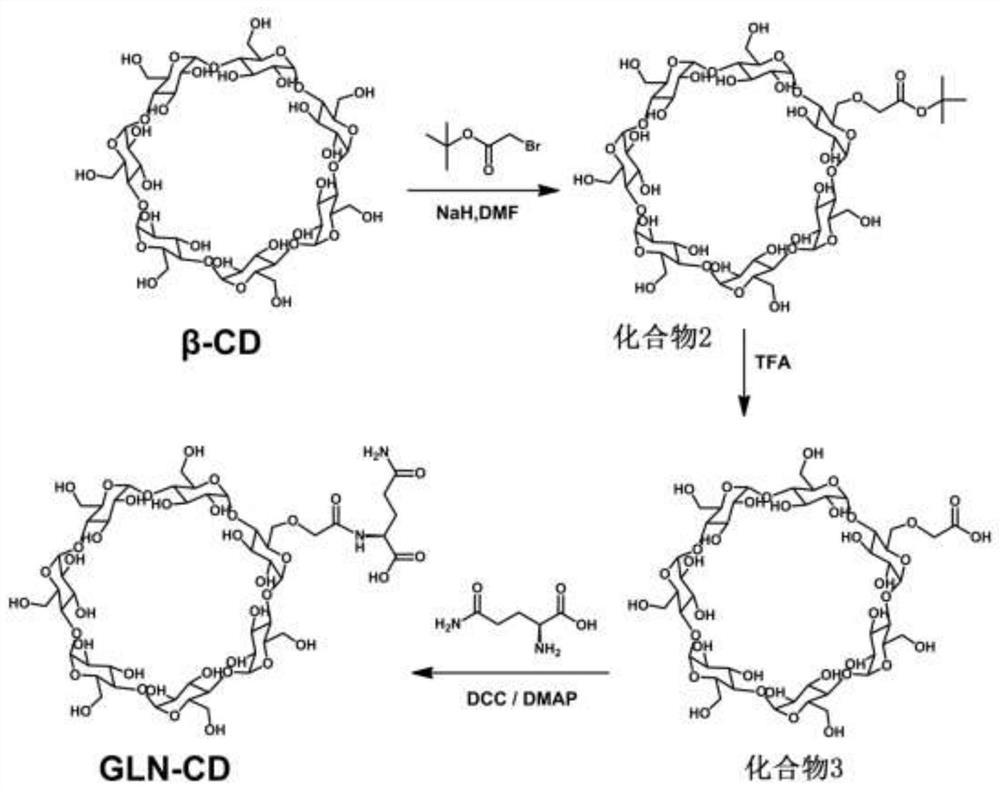
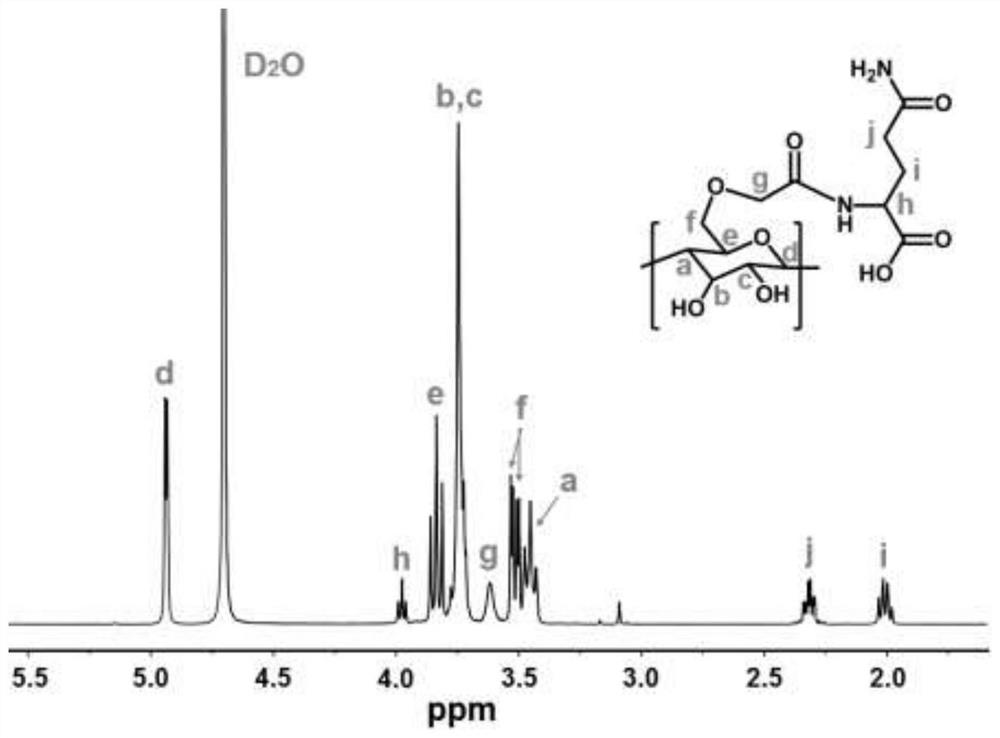
[0063] Example 2: Synthesis of glutamine-cyclodextrin.
[0064] A preparation method of glutamine-modified cyclodextrin, which uses tert-butyl bromoacetate to replace the hydrogen of the α-hydroxyl group of β-cyclodextrin under sodium-hydrogen conditions, and then uses trifluoroacetic acid to remove the tert-butyl bromoacetate. The butyl group forms a free carboxyl group, and finally the free carboxyl group is linked to the amino group of L-glutamine under the action of the condensing agent 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Form amide bonds to make glutamine-modified β-cyclodextrin, and the modification ratio of glutamine to β-cyclodextrin is 1:1 to 7:1, both of which can achieve the drug effect of this scheme. Now the modification ratio Taking 1:1 as an example, the synthesis method is described in detail as follows.
[0065] Specific steps:
[0066] 1) Add sodium hydrogen (4 g, 0.1 mol) and tert-butyl bromoacetate (19.5 g, 0.1 mol), heated to 80°...
Embodiment 3
[0070] Example 3: Glutamine-cyclodextrin and doxorubicin form inclusion complexes.
[0071] Dissolve 100mg GLN-CD (75.7μmol) in 6mL ultrapure water at 40°C, and dissolve 37.8, 50.5, 75.7 and 151.4μmol of doxorubicin (DOX) in 0.6mL DMSO respectively, and then separately Add dropwise to the GLN-CD solution, and continue stirring at 40°C for 8 hours. Precipitate with 80 mL of absolute ethanol and wash 3 times, collect the precipitate and dry it in vacuum to obtain doxorubicin-glutamine-cyclodextrin inclusion compound (DOX@GLN-CD). After doxorubicin enters the inner cavity of cyclodextrin, the fluorescence intensity increases, so the inclusion efficiency of doxorubicin is analyzed by fluorescence spectroscopy, and the test concentration of each inclusion complex is 9.2×10 -4 mol / L, the excitation wavelength is 495nm.
[0072] Such as Figure 5-6 As shown, as the ratio of DOX:GLN-CD increases, the fluorescence gradually increases, indicating that DOX and GLN-CD have successfully...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com