A kind of preparation method of 1,1-diaryl alkane derivative
A technology of diaryl alkanes and derivatives, applied in 1 field, to achieve the effects of excellent functional group tolerance, simple operation and mild reaction conditions
Active Publication Date: 2022-02-25
NANCHANG HANGKONG UNIVERSITY
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
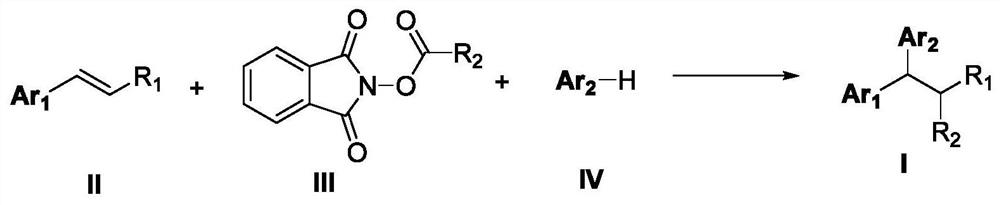
However, current styrene difunctionalization reactions involving alkyl NHP esters mainly focus on the construction of C(sp3)-C(sp3) / C(sp3)-heteroatom bonds, while styrene and (hetero)arenes are bonded via C (sp2)-H breaks, and the alkylation reaction of building C(sp3)-C(sp3) / C(sp3)-C(sp2) bond has not been reported in the prior art
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0033] Below in conjunction with specific embodiment, the present invention is described in further detail. Hereinafter, unless otherwise specified, the methods described are conventional methods in the art, and the reagents used can be purchased through commercial channels.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
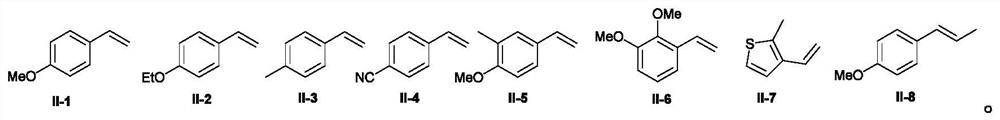
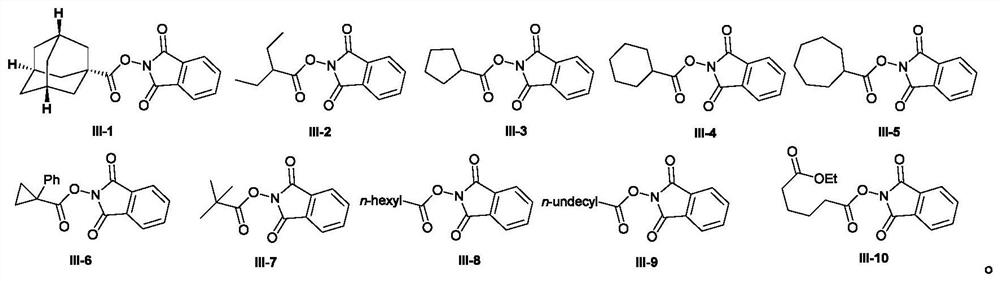
The invention discloses a preparation method of 1,1-diaryl alkane derivatives. The method uses olefin compounds, (hetero) aromatics and N-hydroxyphthalimide ester compounds as raw materials to construct C(sp3)-C(sp3) / C(sp3) under photoredox catalysis conditions. The ‑C (sp2) bond realizes the 1, 2‑difunctionalization reaction of alkenes, and prepares a series of 1, 1‑diaryl alkane derivatives.
Description
technical field [0001] The application belongs to the technical field of organic synthesis, and in particular relates to a preparation method of 1,1-diaryl alkane derivatives. Background technique [0002] Carboxylic acid derivatives-N-hydroxyphthalimide alkyl esters (alkyl NHP esters) have become a very important alkylating agent in organic synthesis, not only by using cheap, readily available and stable Fatty acids are simple to prepare, and can easily generate alkyl radicals by decarboxylation under photoredox catalysis, transition metal catalysis, etc. (1) Org.Lett.2018, 20, 6659-6662; 2) Org.Lett.2018 , 20, 888-891; 3) Org. Lett. 2018, 20, 3496-3499; 4) ACS Catal. 2018, 8, 7489-7494; 5) Org. Lett. 2018, 20, 1546-1549; 6) Org.Lett.2018, 20, 224-227; 6) Chem.Eur.J.2018, 24, 4552-4555; 7) Angew.Chem.Int.Ed.2017, 56, 3708-3711; 9) J. Org. Chem. 2015, 80, 6025-6036; 10) NATURE COMMUNICATIONS, (2018) 9: 5215, etc.). In recent years, alkyl NHP esters have been used by organ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07C41/30C07C213/08C07D207/333C07D209/08C07D209/12C07D209/24C07D409/06C07D471/06C07B37/02C07C217/80C07C43/21
CPCC07D209/12C07D209/24C07D209/08C07D409/06C07D471/06C07D207/333C07C213/08C07C41/30C07B37/02C07C2603/74C07C217/80C07C43/21
Inventor 欧阳旋慧梁云燕宋仁杰李金恒
Owner NANCHANG HANGKONG UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com