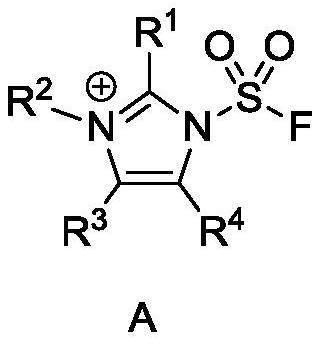
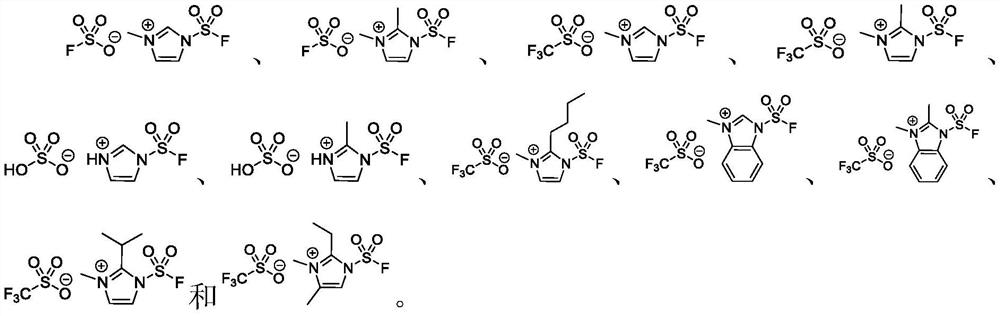
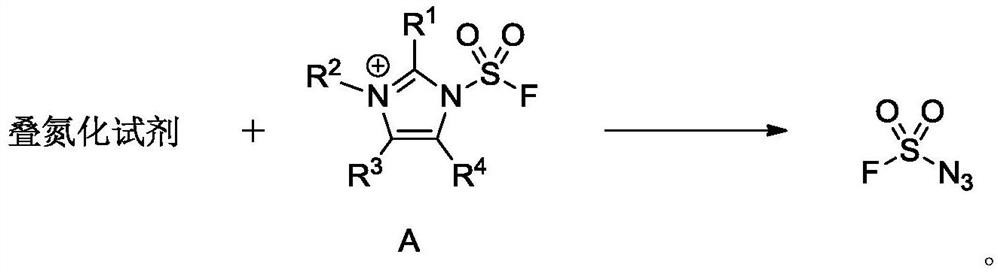
A kind of fluorosulfonyl compound is preparing fso 2 n 3 applications in
A technology of FSO2N3, fluorosulfonyl, applied in the direction of azide acid/azide/halide azide, organic chemistry, etc., can solve the problems of low safety, poor preparation yield, etc., and achieves safe and simple method, high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of 1-(Fluorosulfonyl)-2,3-dimethyl-1H-imidazole trifluoromethanesulfonate
[0051]
[0052] 2-methylimidazole [compound 1] (49.3g, 600mmol) was added to the suspension of sodium carbonate (159.1g, 1500mmol) in acetonitrile (600mL) at room temperature, and the reaction system was pumped to a negative pressure by a water pump and introduced into sulfuryl fluoride with a balloon Gas [compound 2] (18 L, 730 mmol), stirred overnight, TLC (petroleum ether:ethyl acetate=10:1, product R f = 0.44) detected the completion of the reaction, the reaction solution was filtered with silica gel (10-40 mesh), the filter cake was washed with dichloromethane (600 mL), the filtrate was extracted with distilled water (3000 mL×3), and the aqueous phases were combined with dichloromethane (600 mL). ) back extraction, the organic phases were combined and washed with saturated brine (600 mL), dried over anhydrous sodium sulfate, the filtrate was concentrated by rotary eva...
Embodiment 2
[0055] Preparation of 1-(Fluorosulfonyl)-3-methyl-1H-imidazole trifluoromethanesulfonate
[0056]
[0057] To the sodium carbonate (4.2 g, 40 mmol) acetonitrile (80 mL) suspension was added imidazole [compound 5] (1.36 g, 20 mmol) at room temperature, the reaction system was pumped to a negative pressure by a water pump, and then the sulfuryl fluoride gas [compound 2] was introduced with a balloon. ] (0.6L, 25mmol), stirred overnight, TLC (petroleum ether:ethyl acetate=10:1, product R f = 0.48) to detect the completion of the reaction, add water (200 mL) to the system to separate the reaction liquid, extract with dichloromethane (200 mL × 3), combine the organic phases and wash with saturated brine (150 mL), dry over anhydrous magnesium sulfate, and dry over anhydrous magnesium sulfate. The algae is filtered, and the filtrate is concentrated to about 40mL through a rotary evaporator (the boiling point of 1H-imidazole-1-sulfonyl fluoride is lower, and the temperature...
Embodiment 3
[0060] Preparation of 1-(Fluorosulfonyl)-1H-imidazole hydrogen sulfate
[0061]
[0062] To the sodium carbonate (2.1 g, 20 mmol) acetonitrile (40 mL) suspension was added imidazole [compound 5] (0.68 g, 10 mmol) at room temperature, the reaction system was pumped to negative pressure by a water pump, and then the sulfuryl fluoride gas [compound 2] was introduced with a balloon. ] (0.4L, 16mmol), stirred overnight, TLC (petroleum ether:ethyl acetate=10:1, product R f = 0.48) to detect the completion of the reaction, add water (100 mL) to the system to separate the reaction liquid, extract with dichloromethane (80 mL × 3), combine the organic phases and wash with saturated brine (60 mL), dry over anhydrous magnesium sulfate, and dry over anhydrous magnesium sulfate. The algae was filtered, and the filtrate was concentrated to 20mL through a rotary evaporator (the boiling point of 1H-imidazole-1-sulfonyl fluoride was lower, the temperature was controlled below 28°C du...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com