Epidermal growth factor receptor inhibitor and preparation and application thereof
A technology selected from, C1-C8, applied in the field of medicine, can solve the problems of poor selectivity and low tolerance dose of EGFRT790M mutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
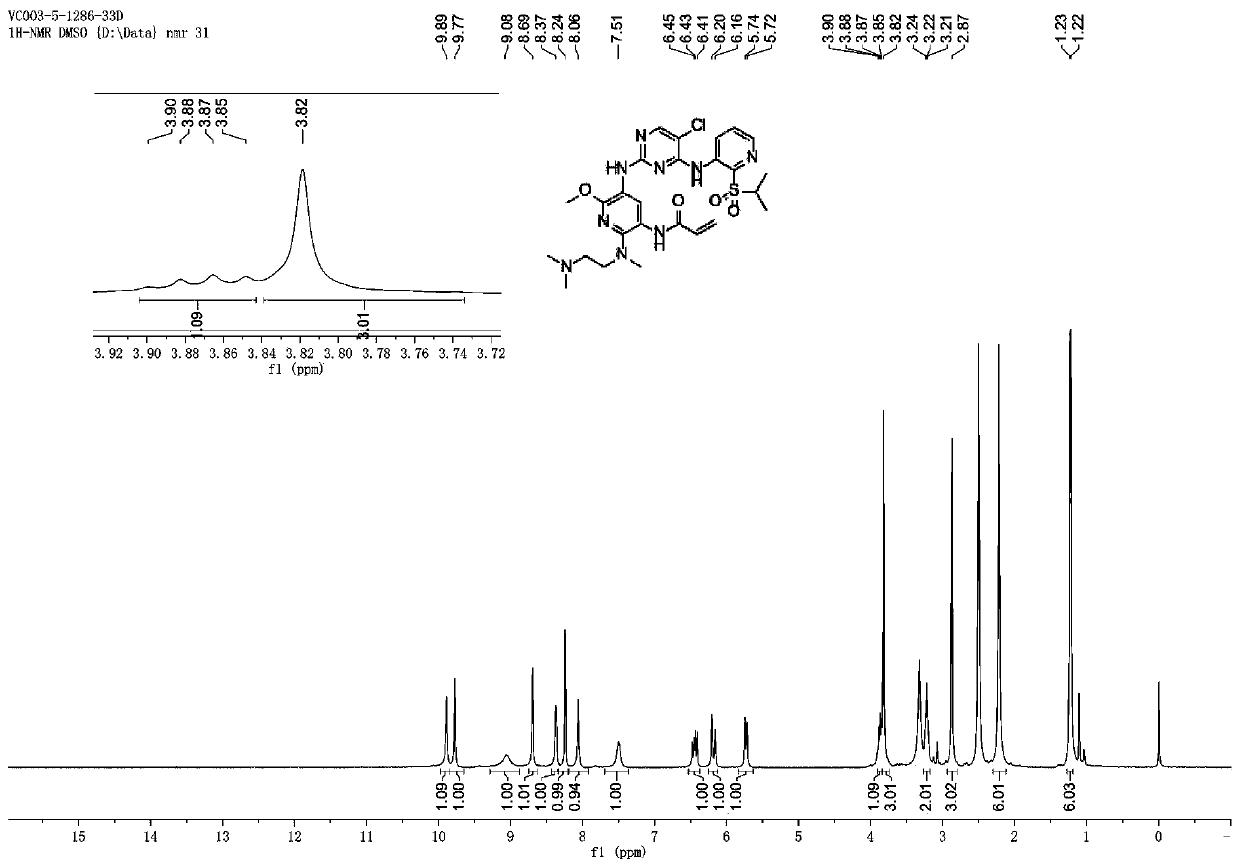
[0114] N-(5-((5-chloro-4-((2-(isopropylsulfonyl)pyridin-3-yl)aminopyrimidin-2-yl)amino)-2-((2-(dimethyl Synthesis of amino)ethyl)(methyl)amino)-6-methoxypyridin-3-yl)acrylamide
[0115]
[0116] Step 1: Synthesis of 6-bromo-2-methoxy-3-nitropyridine
[0117]
[0118] At room temperature, sequentially add 2,6-dibromo-3-nitropyridine (40.00g, 141.90mmol), THF (520ml) into the four-necked flask, cool down to 0-5°C, add sodium methoxide (30%, 28.11g , 156.08mmol), 3h reaction terminated. Pour the reaction solution into ice water (500ml), add MTBE (500ml x3) for extraction, combine the organic phases, wash with saturated brine (200ml), concentrate the organic phases, and crystallize the crude product to obtain 19.89g of a light yellow solid, with a yield of 60% . 1 H NMR (400MHz, Chloroform-d) δ 8.15 (d, J = 8.2Hz, 1H), 7.22 (d, J = 8.2Hz, 1H), 4.14 (s, 3H).
[0119] Step 2: Synthesis of 6-bromo-2-methoxypyridin-3-amine
[0120]
[0121] At room temperature, 6-bromo-2...
Embodiment 2
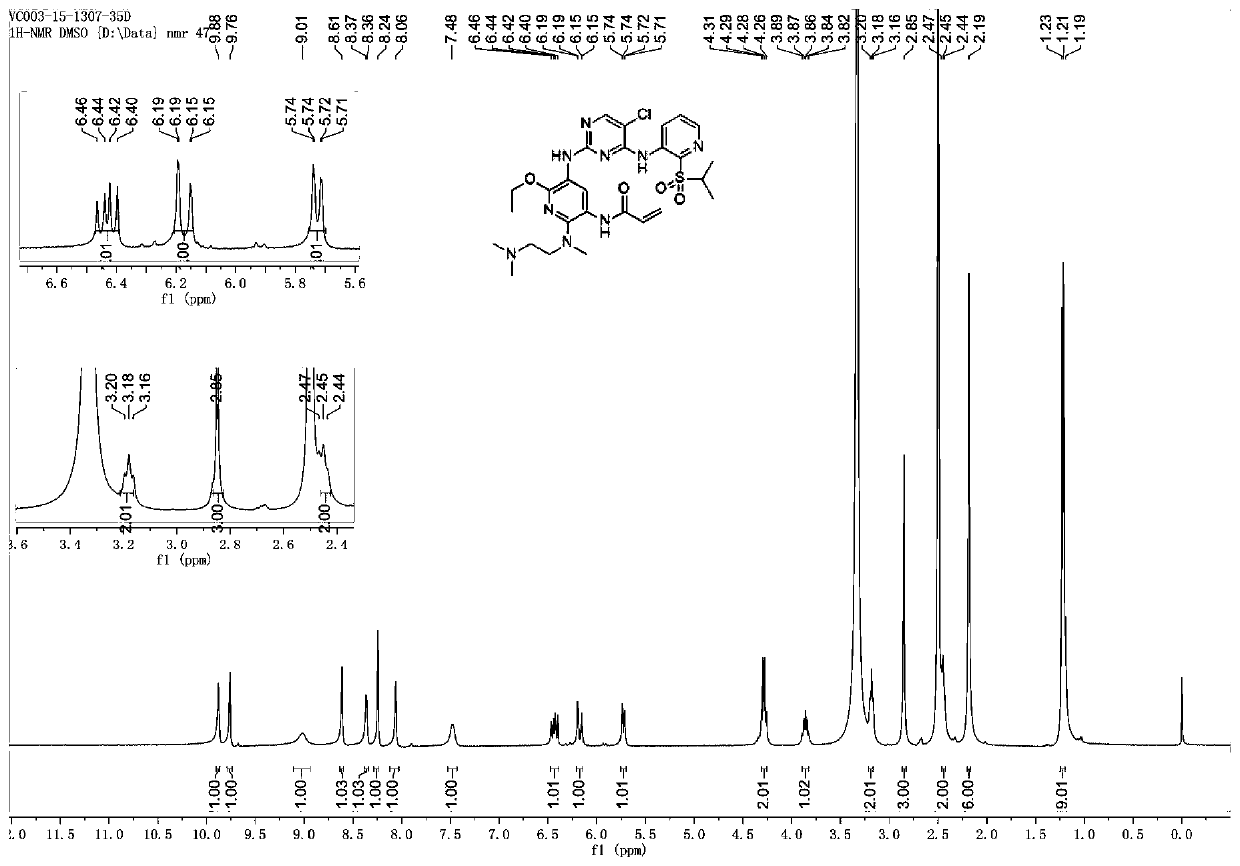
[0156] N-(5-((5-chloro-4-((2-(isopropylsulfonyl)pyridin-3-yl)amino)pyrimidin-2-yl)amino)-2-((2-(dimethyl Synthesis of (amino)ethyl)(methyl)amino)-6-ethoxypyridin-3-yl)acrylamide
[0157]
[0158] N-(5-((5-chloro-4-((2-(isopropylsulfonyl)pyridin-3-yl)amino)pyrimidin-2-yl)amino)-2-((2-(dimethyl The preparation method of (amino) ethyl) (methyl) amino)-6-ethoxypyridin-3-yl) acrylamide is similar to that of Example 1.
[0159] 1 H NMR (400MHz, DMSO-d 6 )8.61(s,1H),8.36(d,J=4.0Hz,1H),8.24(s,1H),8.06(s,1H),7.48(s,1H),6.43(dd,J=17.0,10.2 Hz,1H),6.21-6.14(m,1H),5.75-5.68(m,1H),4.29(q,J=7.0Hz,2H),3.86(dt,J=13.5,6.8Hz,1H),3.18 (t, J=6.3Hz, 2H), 2.85(s, 3H), 2.46(d, J=6.7Hz, 2H), 2.19(s, 6H), 1.23(s, 9H). MS(ESI)m / z:618.1[M+H] + .
Embodiment 3
[0161] N-(5-((5-chloro-4-((2-(isopropylsulfonyl)pyridin-3-yl)amino)pyrimidin-2-yl)amino)-2-((2-(dimethyl Synthesis of (amino)ethyl)(methyl)amino)-6-isopropoxypyridin-3-yl)acrylamide
[0162]
[0163] N-(5-((5-chloro-4-((2-(isopropylsulfonyl)pyridin-3-yl)amino)pyrimidin-2-yl)amino)-2-((2-(dimethyl The preparation method of (amino)ethyl) (methyl)amino)-6-isopropoxypyridin-3-yl)acrylamide is similar to that of Example 1.
[0164] 1 H NMR (400MHz, DMSO-d 6 )9.87(br,1H),9.75(br,1H),8.98(br,1H),8.52(s,1H),8.36(d,J=3.8Hz,1H),8.25(s,1H),8.05( s,1H),7.47(s,1H),6.43(dd,J=17.0,10.2Hz,1H),6.21-6.13(m,1H),5.76-5.69(m,1H),5.14(p,J= 6.1Hz, 1H), 3.85(dt, J=13.8, 6.9Hz, 1H), 3.17(t, J=6.4Hz, 2H), 2.84(s, 3H), 2.46-2.42(m, 2H), 2.18( s, 6H), 1.21 (dd, J = 10.5, 6.5Hz, 12H). MS(ESI)m / z:632.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com