Phosphate of epidermal growth factor receptor kinase inhibitor and preparation method thereof
A phosphate and compound technology, applied in the field of chemical medicine, can solve the problems of dose climbing, achieve low humidity, improve bioavailability, and facilitate industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation method of formula (I) compound phosphate:
[0055] Dissolve 10.2 mg of the compound of formula (I) in 0.9 mL of acetone, add 0.09 mL of 0.2 mol / L phosphoric acid solution dropwise, stir and react at 50°C for 24 hours, and collect the solid to obtain it.
[0056] The phosphate product that above-mentioned method prepares, its 1 H NMR identification data are as follows:
[0057] 1 H NMR (400MHz, DMSO) δ10.17(s,1H),8.67(s,1H),8.28(s,1H),8.10(s,1H),7.74(s,1H),7.51(dd,J= 16.7,8.3Hz,2H),7.26(t,J=8.1Hz,2H),6.61(d,J=2.0Hz,1H),6.44(dd,J=17.0,10.1Hz,1H),6.26(dd, J=17.0, 1.8Hz, 2H), 5.76(dd, J=10.1, 1.9Hz, 1H), 3.77(s, 3H), 3.56(d, J=4.7Hz, 4H), 3.03(d, J=25.7 Hz,4H),2.07(d,J=15.7Hz,3H).
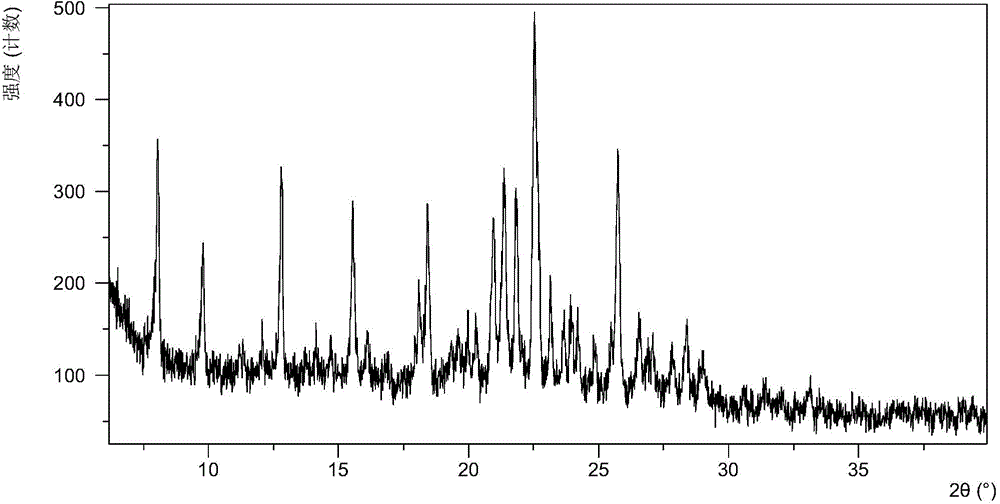
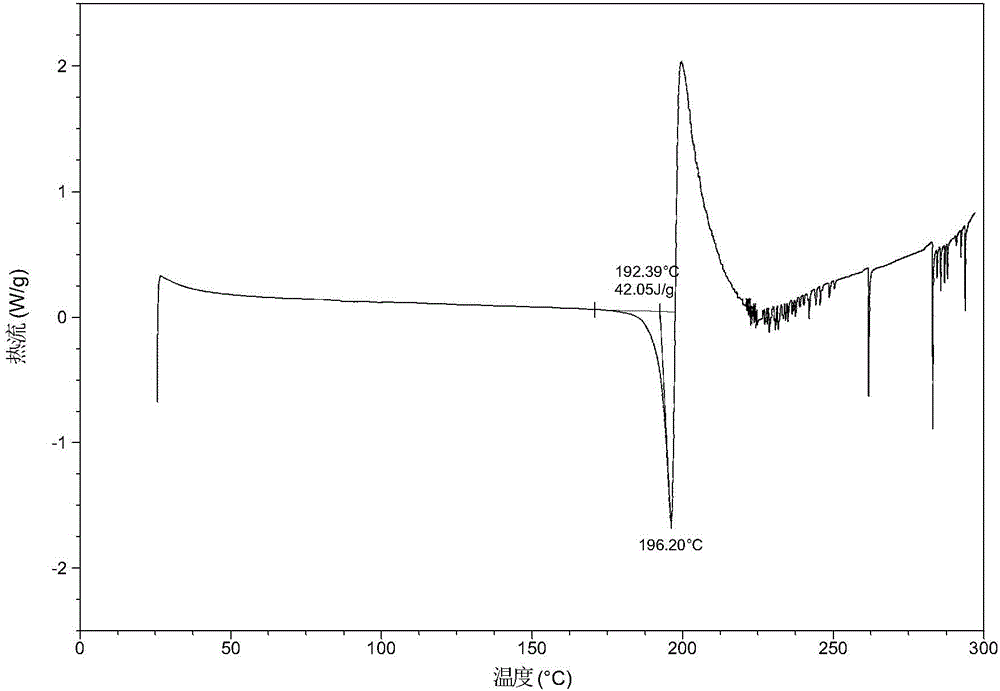
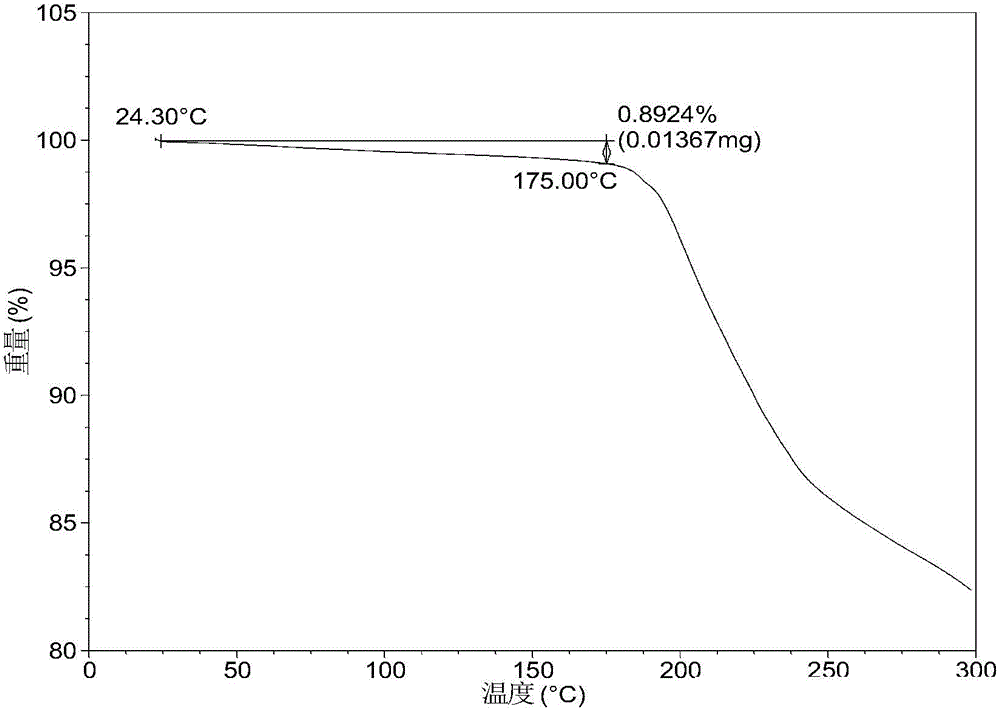
[0058] After testing, the solid obtained in this example is crystal form A, and its X-ray powder diffraction data are shown in Table 1. Its XRPD pattern is as followsfigure 1 , and its DSC graph is shown in figure 2 , and its TGA figure is shown in image 3 ,That 1 H NM...
Embodiment 2
[0063] The preparation method of formula (I) compound phosphate:
[0064] Dissolve 10.2 mg of the free base of the compound of formula (I) in 0.9 mL of tetrahydrofuran, add 0.09 mL of 0.2 mol / L phosphoric acid solution dropwise, stir and react at 50°C for 24 hours, and collect the solid to obtain it.
[0065] After testing, the solid obtained in this example is crystal form A, and its X-ray powder diffraction data are shown in Table 2.
[0066] Table 2
[0067] 2theta
[0068] 28.93
Embodiment 3
[0070] Comparative study on the solubility of phosphate and patent WO2013138502 hydrobromide:
[0071] Phosphate of the present invention and patent hydrobromide sample are formulated into saturated solution with FeSSIF (artificial intestinal fluid under the full stomach state) and water respectively, after 1 hour, after 4 hours, measure the amount in the saturated solution by high performance liquid chromatography content of the sample. The experimental results are shown in Table 3.
[0072] table 3
[0073]
[0074] As can be seen from the above comparison results, after being placed in FeSSIF and water for 1 hour, the phosphate of the present invention has a higher solubility than the patented hydrobromide after 4 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com