Levodopa formulations for rapid relief of Parkinson's disease
一种左旋多巴、帕金森病的技术,应用在用于快速缓解帕金森病的左旋多巴制剂领域,能够解决不良肠摄取等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] overview
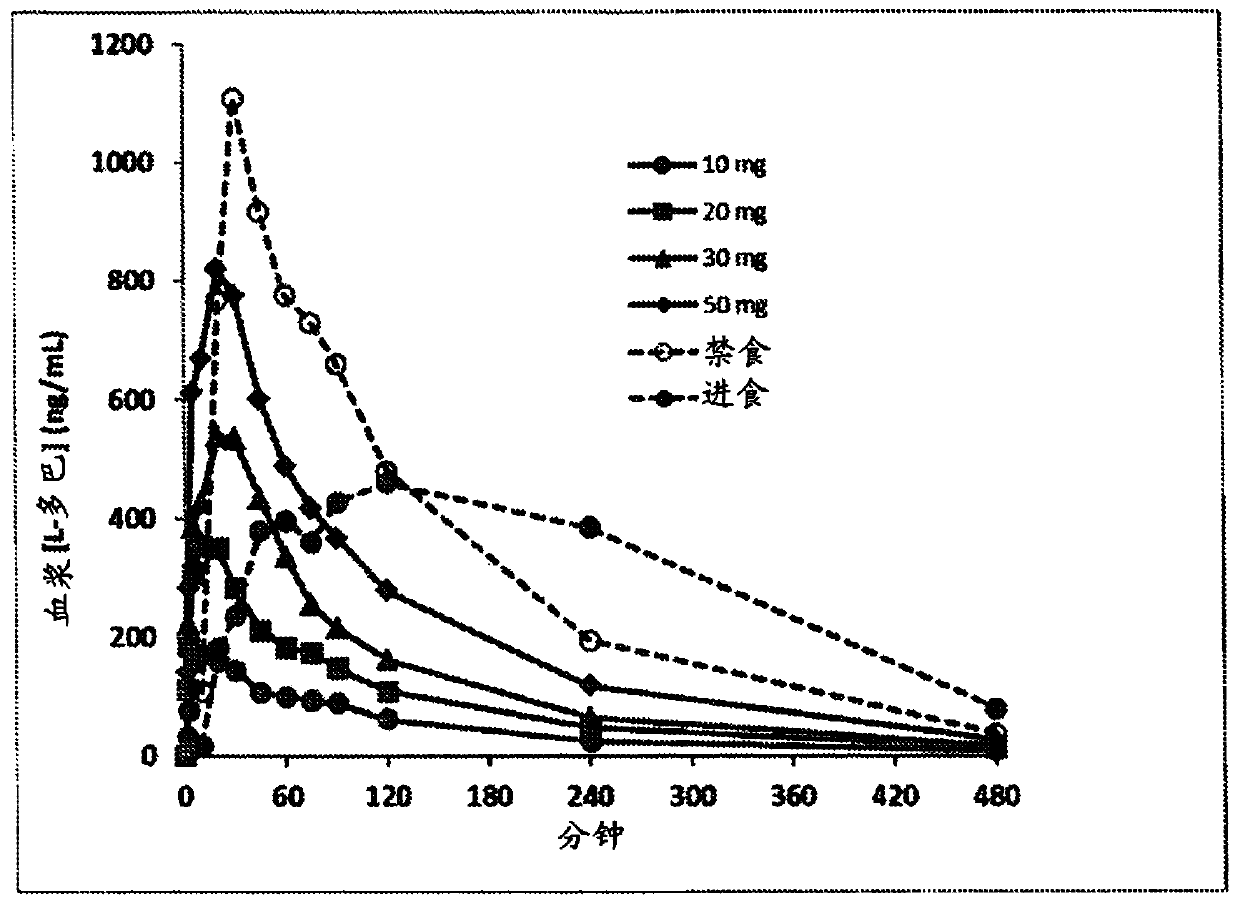
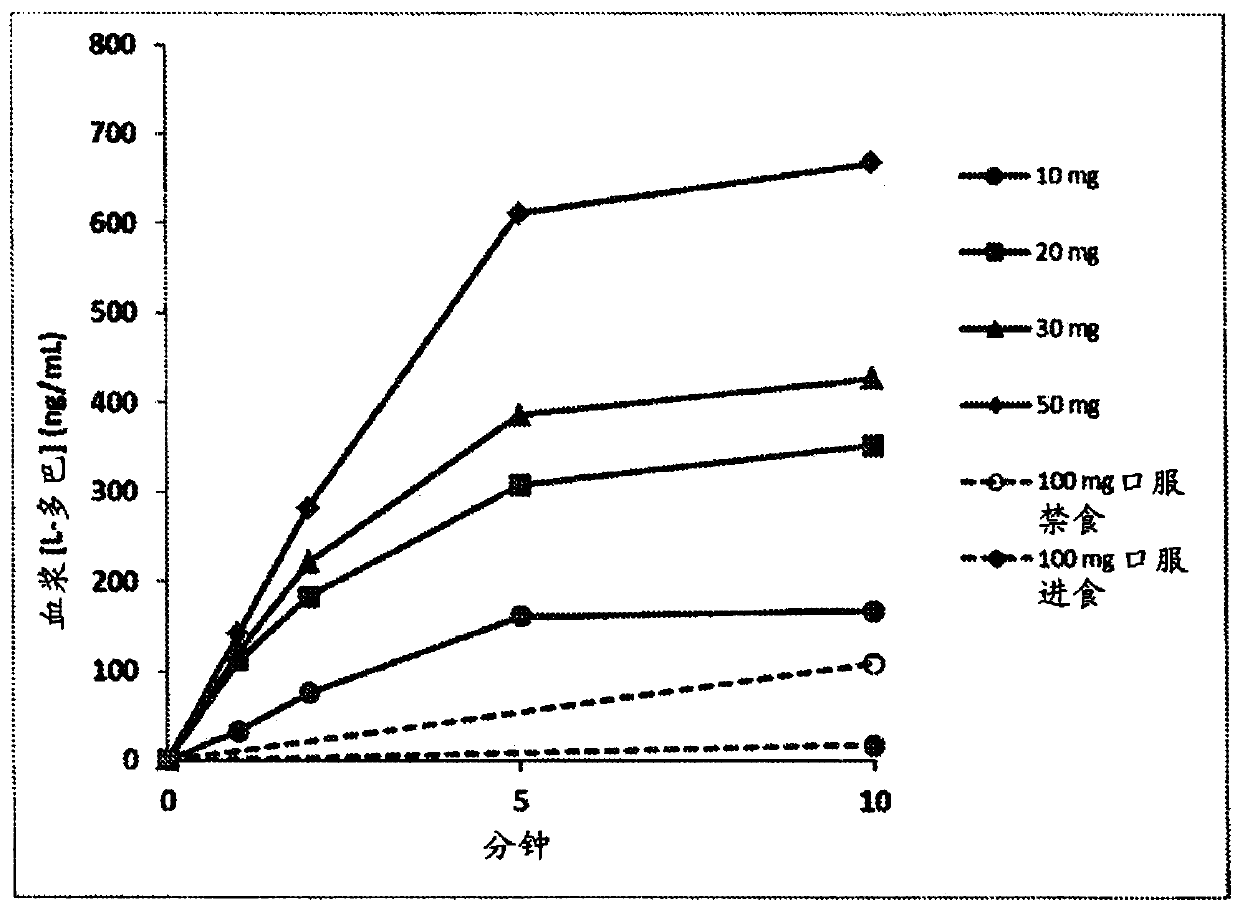
[0102] The 90 / 8 / 2 dry powder levodopa formulation is provided to evaluate the safety, tolerability, and Dopa Pharmacokinetics (PK). The pulmonary levodopa powder described in these examples consisted of 90% levodopa granules, 8% dipalmitoylphosphatidylcholine, and 2% sodium chloride (all on a dry basis) and is referred to herein as "90 / 8 / 2". This data provides an indication of the PK of levodopa following a single inhaled 90 / 8 / 2 dose, compared to oral levodopa (LD) administered under fasted and fed conditions, and with and without carbidol. Comparison of PK in the case of bar (CD) pretreatment. This was a two-part study in healthy adult male and female subjects, as follows: Part A - dose escalation segment compared to oral levodopa; and Part B - 90 / 8 / 2 plus or minus carb Dopa pretreatment link.
[0103] Part A is an open-label, 3-period crossover, single dose escalation study. Each subject received a single oral dose of CD / LD (25 / 100 mg) in the fed or...
Embodiment 2
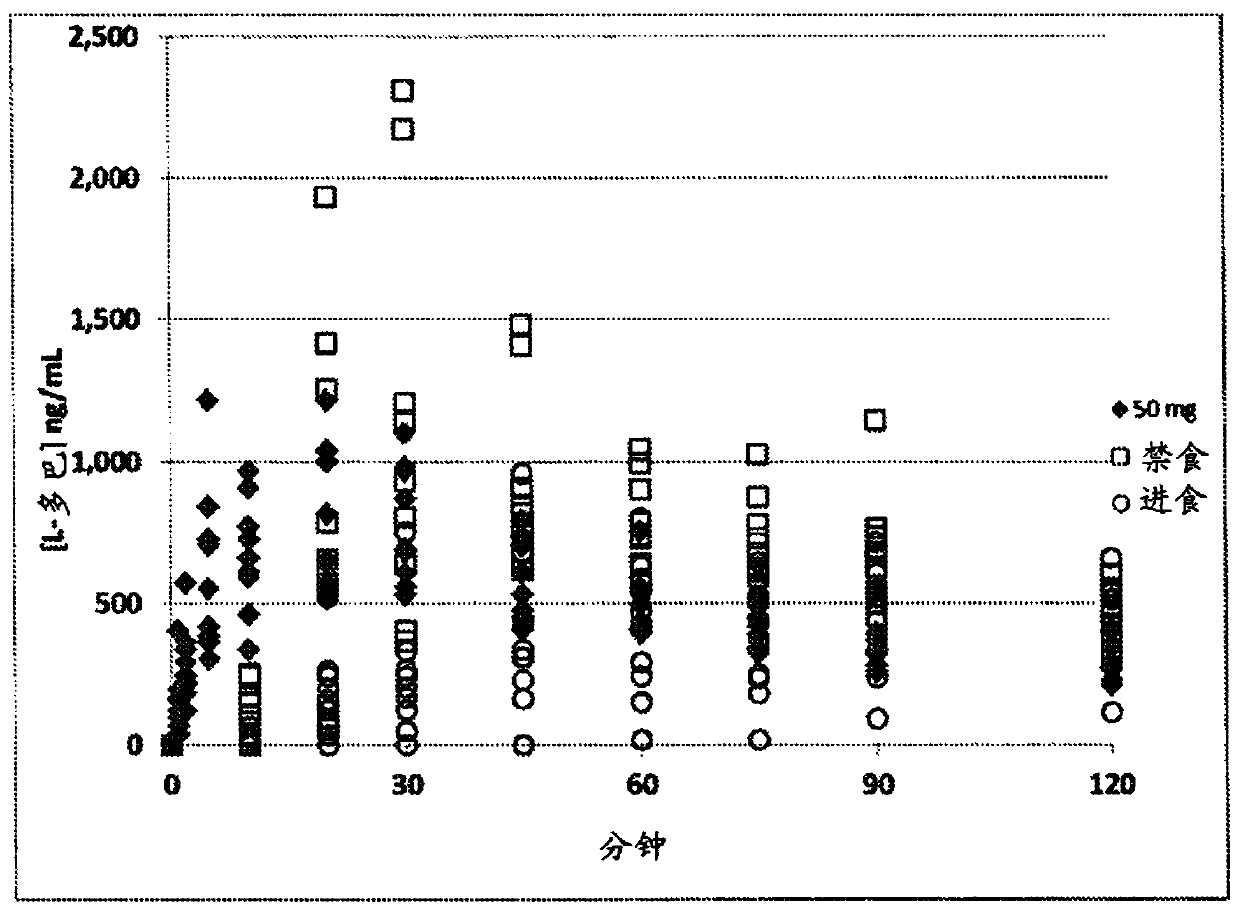
[0193]A phase 2 study testing two doses of pulmonary levodopa (25 mg and 50 mg of study drug) was a multicentre, randomized, double-blind, placebo-controlled, single-dose, with three arms (placebo, 25 mg, and 50 mg ) and included an "open-label" oral Sinemet arm. Twenty-four (24) patients treated in this study were serially assessed for L-dopa plasma levels, exercise response, and safety at each visit. Patients in the off state are administered study drug and serial assessments begin prior to dosing and continue for up to 180 minutes after dosing. Motor function was assessed using the tap test, the Unified Parkinson's Disease Rating Scale Part III (UPDRS III), and subjective assessments of "meaningful" on and off. Safety parameters monitored included pulmonary function, clinical laboratory data, EGC, and vital signs (blood pressure, heart rate, and orthostatic blood pressure). This study was designed to measure the timing, magnitude, and persistence of pulmonary levodopa's e...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap