A circular RNA detection kit predicts responsiveness to neoadjuvant chemotherapy in triple-negative breast cancer
A triple-negative breast cancer, kit technology, applied in the field of biomedicine, can solve the problems of patients' physical decline, increase in lesions, loss of radical tumor treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Composition of the kit for predicting neoadjuvant chemotherapy response to triple-negative breast cancer (50 responses)
[0015] 1. 10ml of DEPC water or enzyme-free water, 10ml of double distilled water;
[0016] 2. Trizol 50ml;
[0017] 3. Trichloromethane 100ml;
[0018] 4. Isopropyl alcohol 100ml;
[0019] 5. 1ml of 5× reverse transcription buffer;
[0020] 6. 1ml of 25mM magnesium chloride;
[0021] 7. 1ml of 10mM base triphosphate deoxynucleotide;
[0022] 8. 5U / μl RAN enzyme inhibitor 500μl;
[0023] 9. 200U / μl MMLV reverse transcriptase 50μl or 25U / μl AMV enzyme 50μl;
[0024] 10. 2x real-time quantitative PCR buffer 2ml;
[0025] 11. 5U / μl Taq polymerase 50μl;
[0026] 12. 50μl of 5μM hsa_circRNA_038632 specific PCR primer;
[0027] (1) Its forward primer is 5'-AGAGCTGCATCATCCTTGCA3' (SEQ ID NO: 2),
[0028] (2) Its reverse primer is 5'-TCTTGTGCAGCTCCAGGAGA-3' (SEQ ID NO: 3).
[0029] 13. 5μM β-actin specific PCR primers 30μl
[0030](1) I...
Embodiment 2
[0034] Example 2 Detection of hsa_circRNA_038632 in tissue samples
[0035] 1. Extract tissue RNA
[0036] Take the tissue specimen and add liquid nitrogen to the mortar to grind the specimen; add 0.6ml Trizol to the mortar, grind it into a homogenate and add it to the tube tube with a spoon; add 0.4ml Trizol to the tube tube; add 200 μl / ml Trizol of chloroform In the tube, shake by hand for 15-30s, place on ice for 5min, centrifuge at 12000g at 4°C for 15min; carefully take the upper aqueous phase into a new tube, add pre-cooled isopropanol 0.5ml / ml Trizol, mix well, and freeze at -20°C Let stand for 20min, centrifuge at 12000g at 4℃ for 10min; discard the supernatant, add 1-2ml of ethanol diluted with 75% DEPC water, mix well, centrifuge at 7500g at 4℃ for 5min, discard the supernatant as much as possible, dry at room temperature for 5-10min, add DEPC water for 10- 20 μl of solubilized RNA. The concentration and quality of RNA were measured by spectrophotometer, the OD260 / ...
Embodiment 3
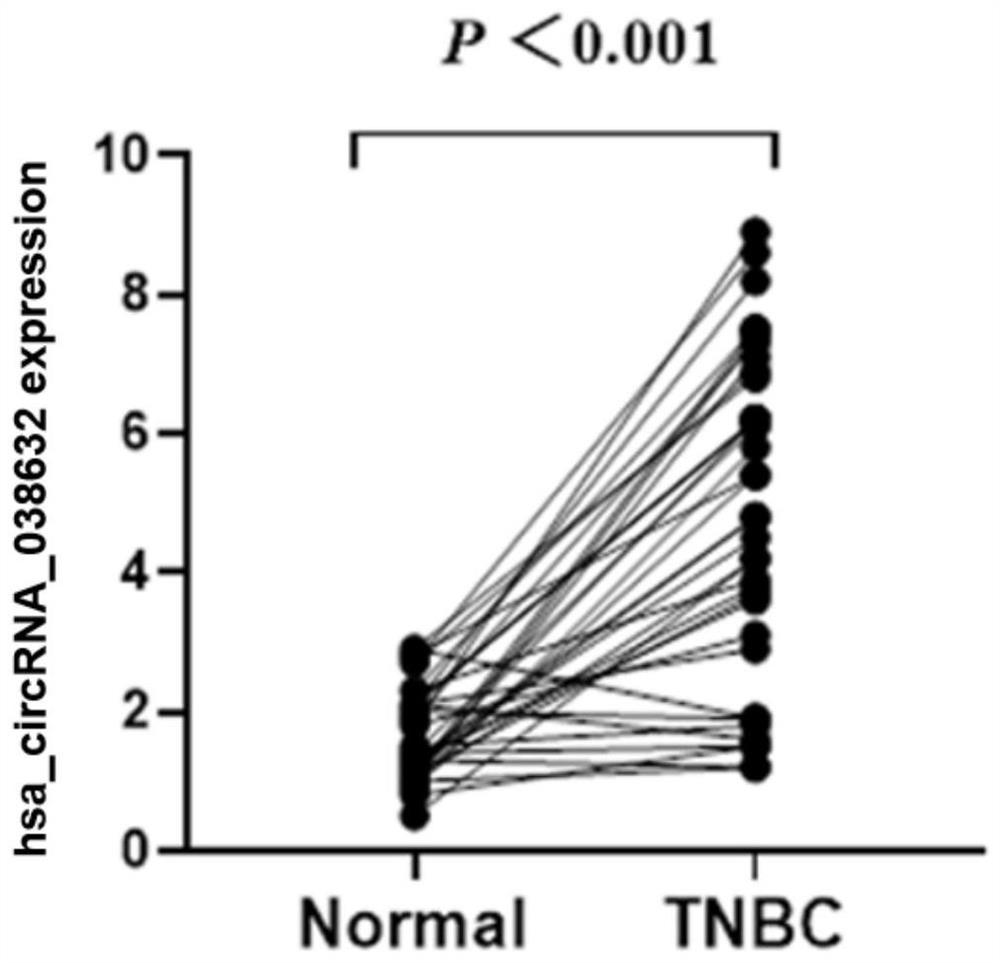
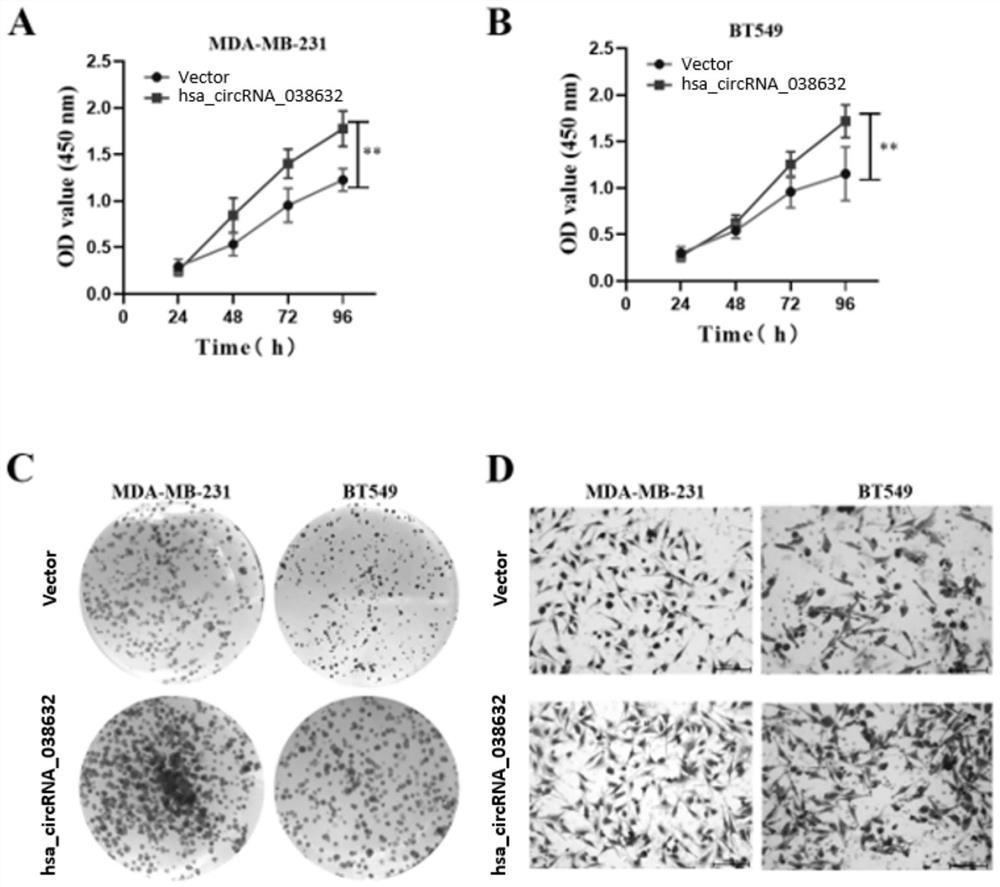
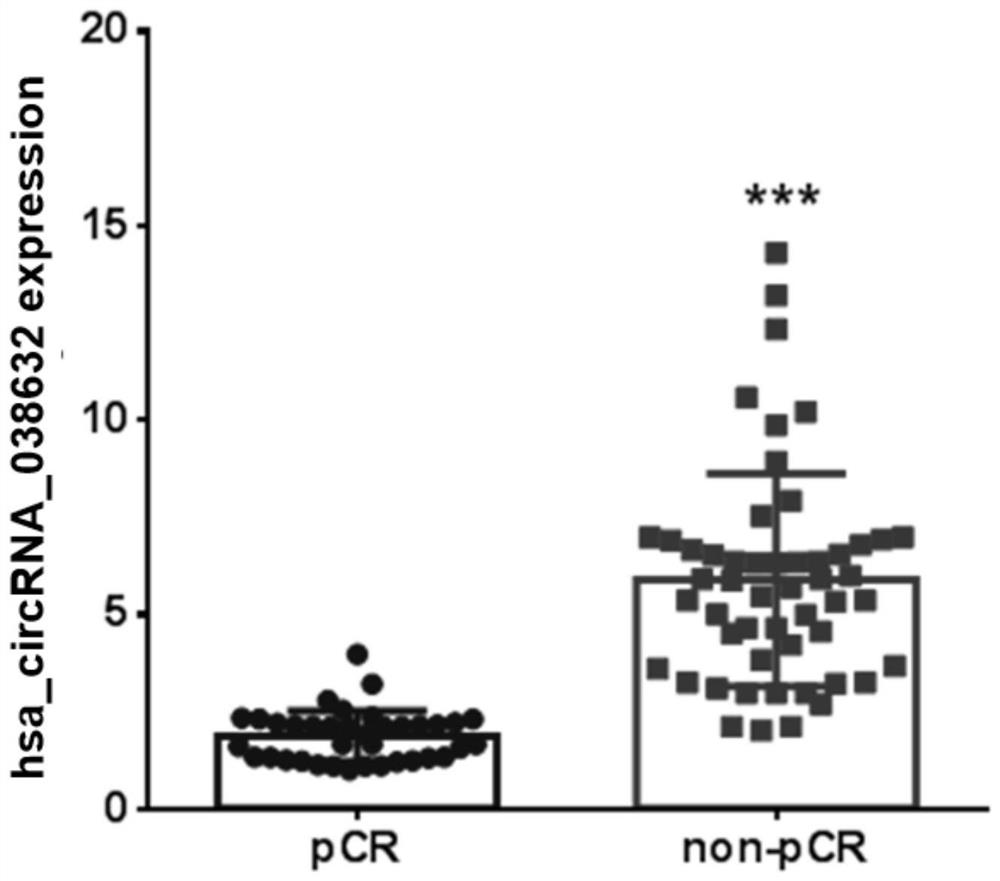
[0049] Example 3 The relationship between hsa_circRNA_038632 and neoadjuvant chemotherapy response in triple-negative breast cancer patients
[0050] The inventors detected the expression of hsa_circRNA_038632 in the tumor tissues of 89 triple-negative breast cancer patients who received neoadjuvant chemotherapy before treatment, and then performed postoperative pathological evaluation for each triple-negative breast cancer patient detected. According to the International Union Against Cancer (UICC) general efficacy evaluation criteria for solid tumors. Patients with response pCR were defined as chemotherapy-responsive, and non-pCR patients were defined as chemotherapy-ineffective / insensitive. The included patients were all female, all of them were diagnosed with invasive breast carcinoma by hollow needle aspiration, and the pathological immunohistochemical results showed that they were triple negative. Imaging tests confirmed no distant metastasis. The neoadjuvant chemother...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com