Zinc-cobalt bimetal cyanide catalyst modified by mixed acid and preparation method thereof
A double metal cyanide and catalyst technology, applied in the field of chemistry, can solve the problems of consumption, quenching of catalyst active sites, reduction of carbon dioxide fixation rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0083] Preparation of zinc-cobalt DMC catalyst
[0084] The parameters are shown in Table 1
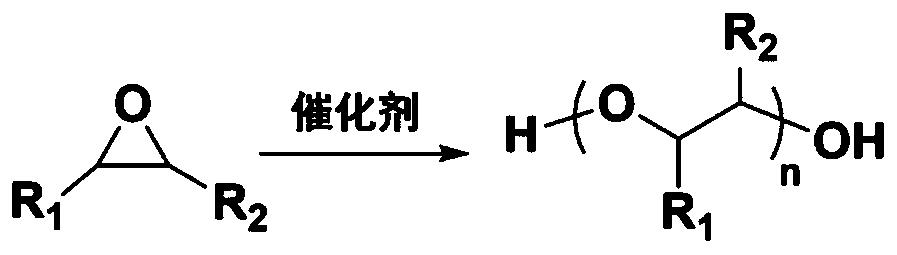
[0085] Weigh a certain amount of cobalt salt and zinc salt, dissolve them in an aqueous solvent, and continue stirring. Add inorganic acid and organic acid, stir for several hours at temperature i), and precipitation continues to form. The turbid liquid is suction filtered and dried to obtain a filter cake. Use an aqueous solvent to re-slurry and wash the filter cake at temperature ii). After stirring for several hours, filter and dry to obtain a filter cake. Repeat the steps of slurrying, washing and drying at temperature ii) until the pH of the system liquid is 6 ~7. The solid product is further dried under vacuum conditions at 80-100°C to obtain the final catalyst, and the catalyst is processed into powder particles by mechanical grinding under anhydrous drying conditions before use.
[0086] Table 1
[0087]
[0088]
Embodiment 9~18
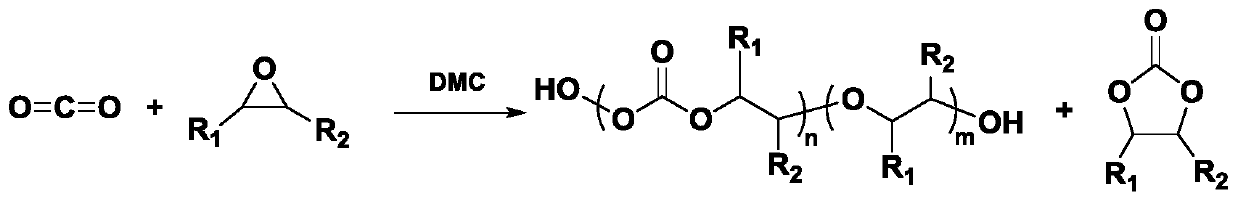
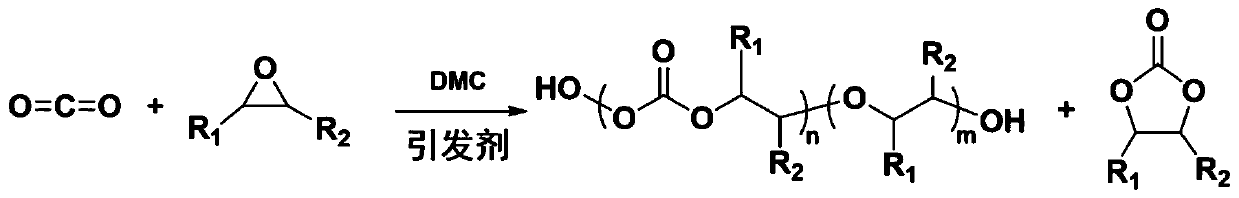
[0090] All reaction conditions, parameters and product parameters are shown in Table 2. The reaction steps are summarized as follows:
[0091] Use the DMC catalyst prepared in Examples 1 to 8, suspended in the initiator and epoxide in a premix container, so that the specified catalyst concentration is reached in the mixed solution, and the components are mixed for a certain period of time at the specified temperature and pressure , The mixture did not react. The mixed suspension is pumped from the mixer to the continuous reactor at a suitable flow rate. The continuous reactor is controlled at the specified reaction temperature and pressure. Each component stays in the continuous reactor for the specified retention time. Collect the obtained products (polycarbonate polyether polyol, cyclic propylene carbonate and unreacted epoxide) in a container, and perform hydrogen NMR characterization of the crude product to calculate the ratio of polymer to cyclic small molecules in the cru...
Embodiment 17
[0097] The proton nuclear magnetic resonance spectrum of the product of Example 17 is as Figure 4 As shown, the gel permeation chromatogram of the polymer product of Example 17 is as Figure 5 Shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com