Intrasite administration and dosing methods and pharmaceuticals for use therein
A drug and biological technology, applied in chemical instruments and methods, separation methods, drug delivery, etc., can solve problems such as insecurity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
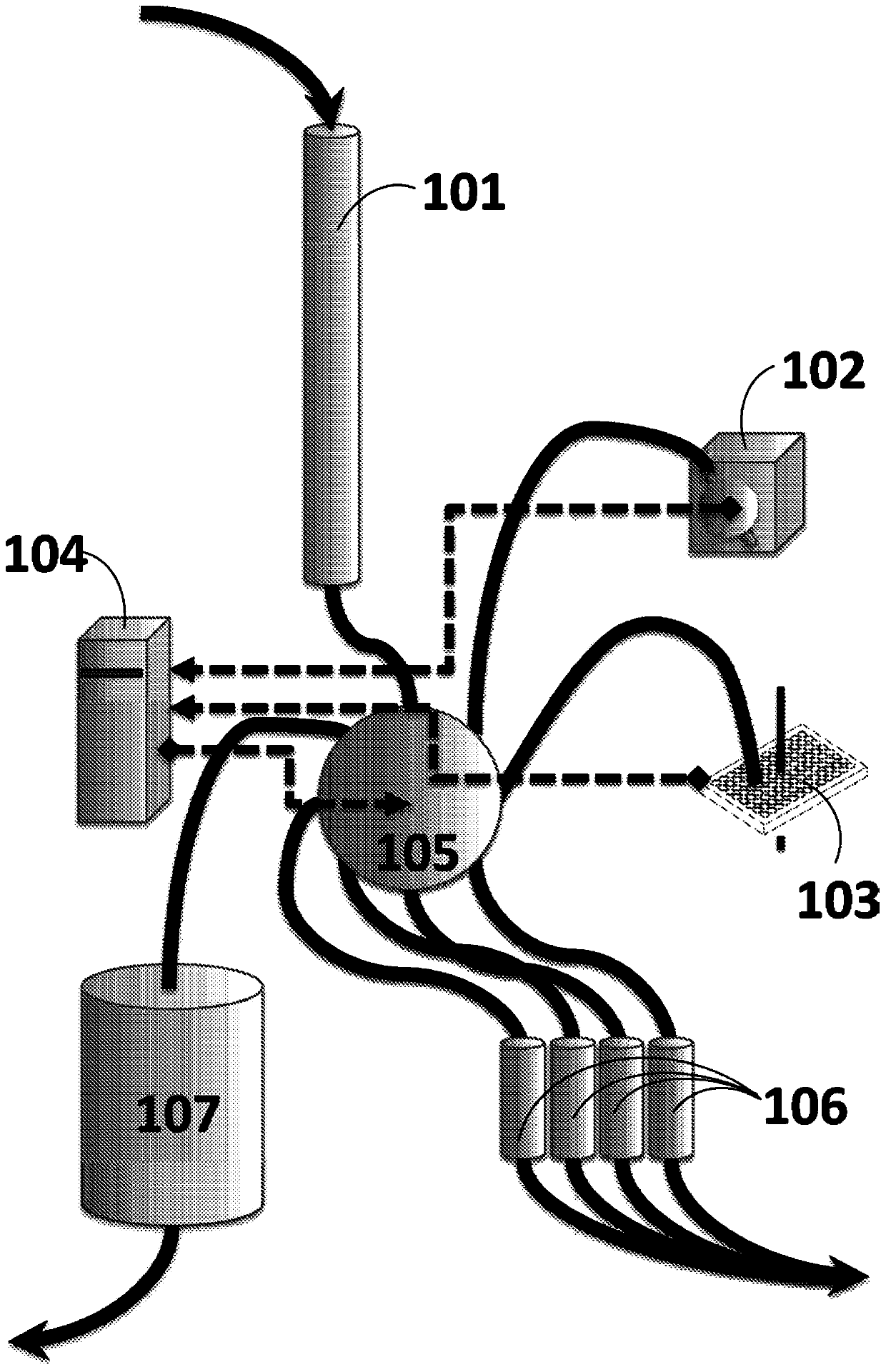
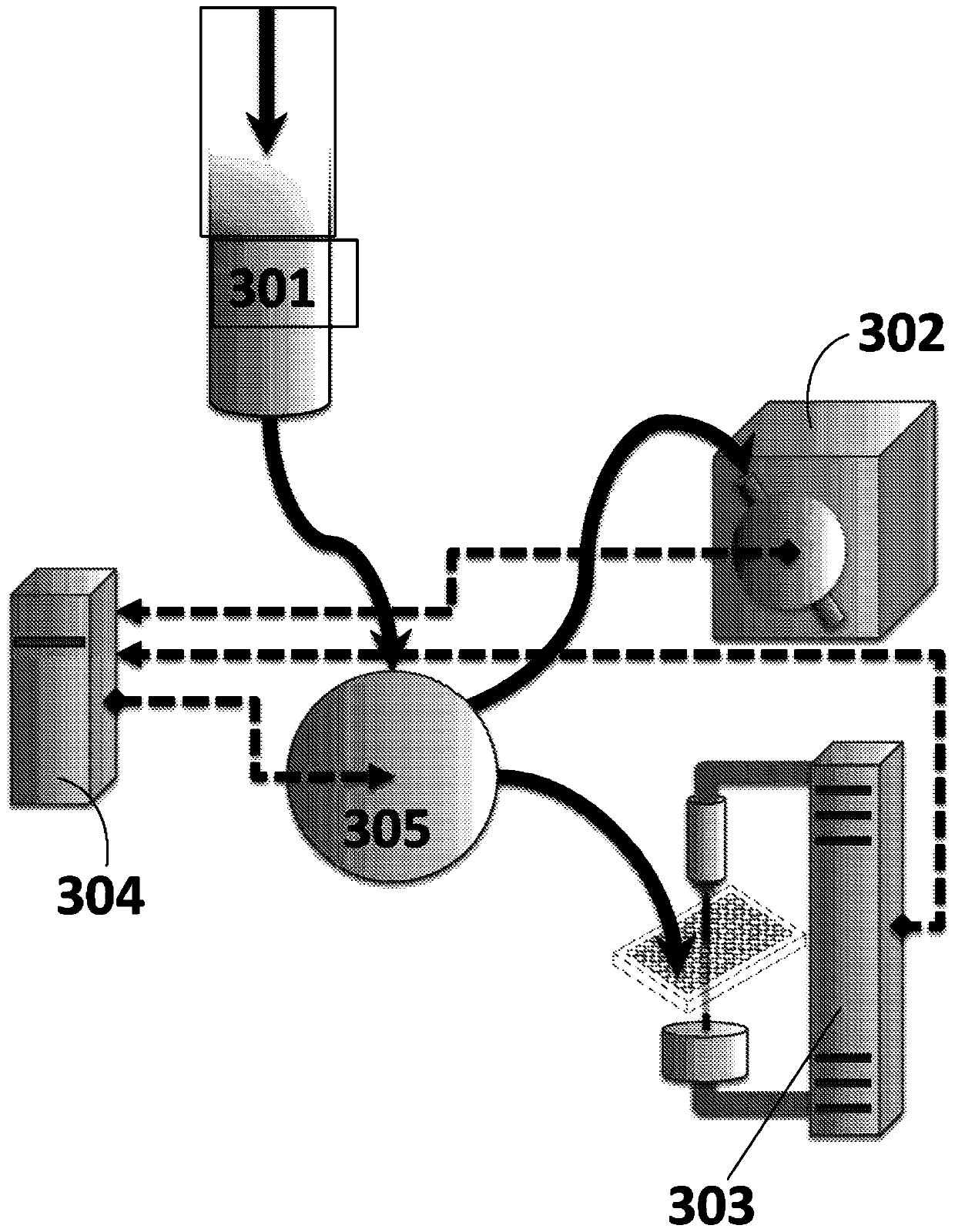
[0170] In-site vancomycin pharmacodynamics test. A single-dose cohort (cohort) pharmacodynamic study was conducted under FDA IND #117494, the first FDA IND approved for intrasite vancomycin. This dose cohort involved the administration of low doses of intrasite lyophilized vancomycin to complex instrumented spinal surgery wounds in adult subjects. Vancomycin and endotoxin levels were measured in the bloodstream and in wound effusion fluid (through wound drains) at specific time points after surgery. Endotoxin levels in the blood stream and effusion fluid were also measured at the same time points after surgery. During administration of IS vancomycin, certified N-90 respirators were required to be worn by all present to prevent inhalation of aerosolized vancomycin powder, a known safety risk for pulmonary fibrosis.
[0171] Intrasite administration of vancomycin. Intrasite administration is based on the surface area of the wound (tissue within the spinal wound deep below t...
Embodiment 2
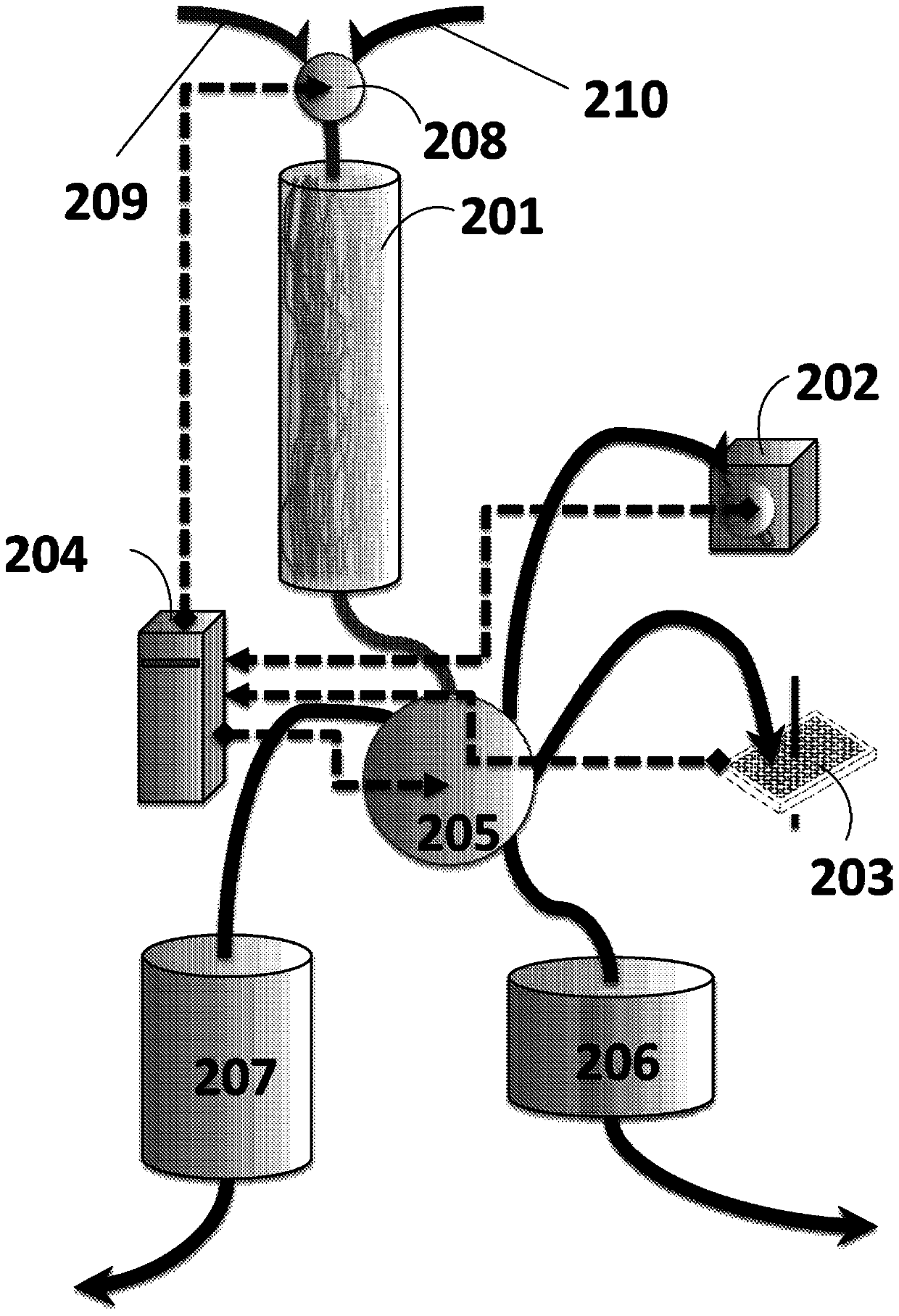
[0186] Endotoxin is removed from vancomycin and / or other IS drugs by ultrapurification. To render a drug suitable for IS administration, various purification methods can be used alone or in series to achieve the desired reduction in endotoxin levels of approximately 10-fold over those found in current vancomycin formulations. Various embodiments of these methods are described herein. To achieve industrial-scale production of endotoxin-depleted vancomycin, vancomycin-rifaximin, or any other IS drug that requires this process, a series of cost-effective and scalable experiments were performed to determine which process or Process combinations are best suited for industrial production / commercial scale production of specific drug products. This scale-up process uses sub-batch confirmatory batch testing to determine endotoxin levels and other impurities based on the methods disclosed herein. Commercial-scale production methods for endotoxin-removed vancomycin, vancomycin-rifaximi...
Embodiment 3
[0188] Testing for endotoxin levels in ultrapure pharmaceuticals. A number of methods exist for the quantitative measurement of endotoxin presence. The most commonly used is the Quantitative Limulus Amebocyte Lysate (qLAL) test, but newer and potentially more accurate and precise methods exist, including gas chromatography / mass spectrometry (GC / MS), high-pressure liquid chromatography / tandem mass spectrometry (HPLC / MS / MS) and human endothelial cell E-selectin binding assay. We compared the accuracy, precision and assay reliability of these methods against standard positive and negative controls on (purified) drug samples to detect endotoxin concentrations down to 0.01 EU / mL. A test method capable of performing accurate and precise down to 0.01EU / mL (indicating the most cost-effectiveness) was used to test our drug samples for endotoxin levels in the future.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap