Gene treatment medicine for hyperuricemia
A technology of gene medicine and uric acid, applied in gene therapy, drug combination, microorganism, etc., can solve the problem of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
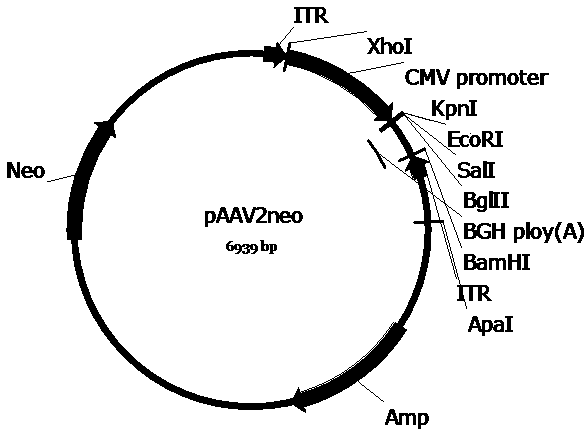
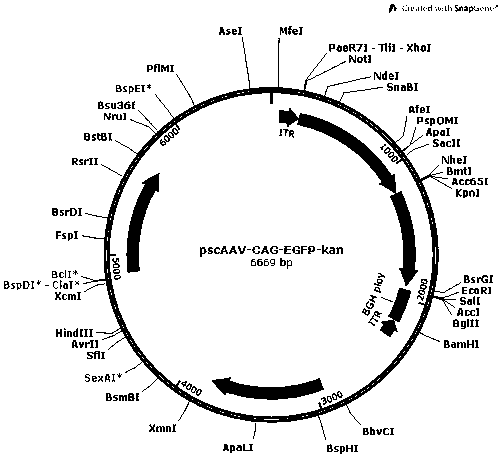
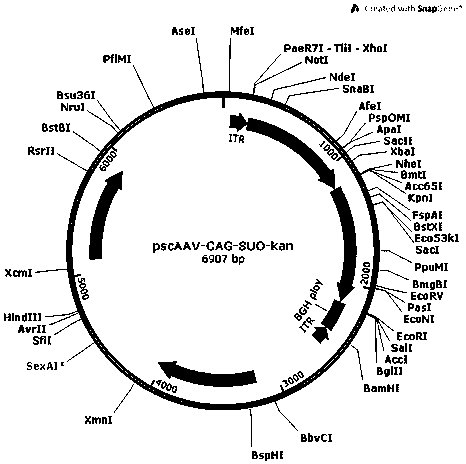
[0062] Example 1 Plasmid vector construction
[0063] In order to construct the pscAAV-CAG-SUO and pscAAV-CAG-SPEG plasmids required for packaging recombinant AAV viruses, we first replaced the pAAV2neo vector with the self-designed CAG promoter (SEQ ID No.9) based on the pAAV2neo preserved by the company In the CMV promoter, replace one side of the ITR sequence in the pAAV2neo vector with a mutated ITR sequence (named ΔITR) (SEQ ID No.13) that deletes the trs (terminal resolution site) and D sequence in the ITR of AAV2, and obtains pscAAV-CAG vector. Next, the artificially synthesized SUO sequence and SPEG sequence were cloned between the KpnI and EcoRI double restriction sites of the pscAAV-CAG vector to obtain the pscAAV-CAG-SUO vector and the pscAAV-CAG-SPEG vector.
[0064] (1) Construction of pscAAV-CAG-SUO vector
[0065] A human-designed secretory urate oxidase sequence SUO. The secretion signal peptide of SUO protein is derived from human Cystatin S protein (SEQ ID...
Embodiment 2
[0068] Example 2 Preparation and assay of recombinant AAV virus
[0069] References (Xiao X, et al . J Virol. 1998;72(3):2224-2232.), the three-plasmid packaging system was used to package the recombinant AAV virus, and the cesium chloride density gradient centrifugation method was used to separate, purify and package the AAV virus. Briefly, AAV vector plasmids (pscAAV-CAG-EGFP, pscAAV-CAM-AUO, pscAAV-CAG-SUO, pscAAV-CAG-SPEG), helper plasmids (pHelper) and AAV Rep and Cap protein expression plasmids (pAAV-R2C1 , pAAV-RC, pAAV-R2C6, pAAV-R2C8 or pAAV-R2C9) were mixed according to the molar ratio of 1:1:1, and transfected into HEK293 cells by the calcium phosphate method. After 48 hours of transfection, the cells and culture supernatant were harvested , application of cesium chloride density gradient centrifugation to separate and purify the recombinant AAV virus. Packaged and purified to obtain scAAV1-CAG-EGFP, scAAV1-CAM-AUO, scAAV1-CAG-SUO, scAAV1-CAG-SPEG, scAAV2-CAG-EGFP...
Embodiment 3
[0075] Example 3 Detection of urate oxidase concentration in the supernatant of recombinant virus infected cells
[0076] 293T cells, primary hepatocytes and MSC cells were mixed at 1×10 6 One per well was spread in a 6-well plate, and four different recombinant AAV viruses scAAV2-CAG-EGFP, scAAV2-CAM-AUO, scAAV2-CAG-SUO, scAAV2-CAG-SPEG were used at MOI=10 5 infected cells. The supernatant was collected 72 hours after infection, and the content of urate oxidase in the serum was measured using the urate oxidase detection kit Amplex Red Uric Acid / Uricase Assay Kit (Life technologies). For the operation process, refer to the kit instruction manual. from Figure 5 It can be seen from the results that, compared with cells infected with scAAV2-CAG-EGFP virus, the supernatant of cells infected with scAAV2-CAM-AUO, scAAV2-CAG-SUO, and scAAV2-CAG-SPEG viruses all have the expression of uric acid oxidase , and the expression of the scAAV2-CAG-SUO and scAAV2-CAG-SPEG groups is better...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap