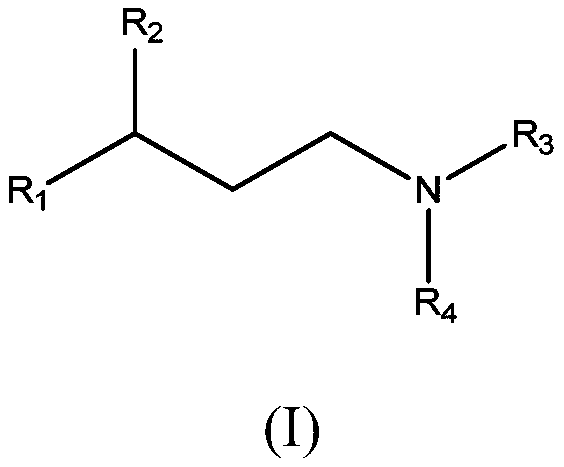
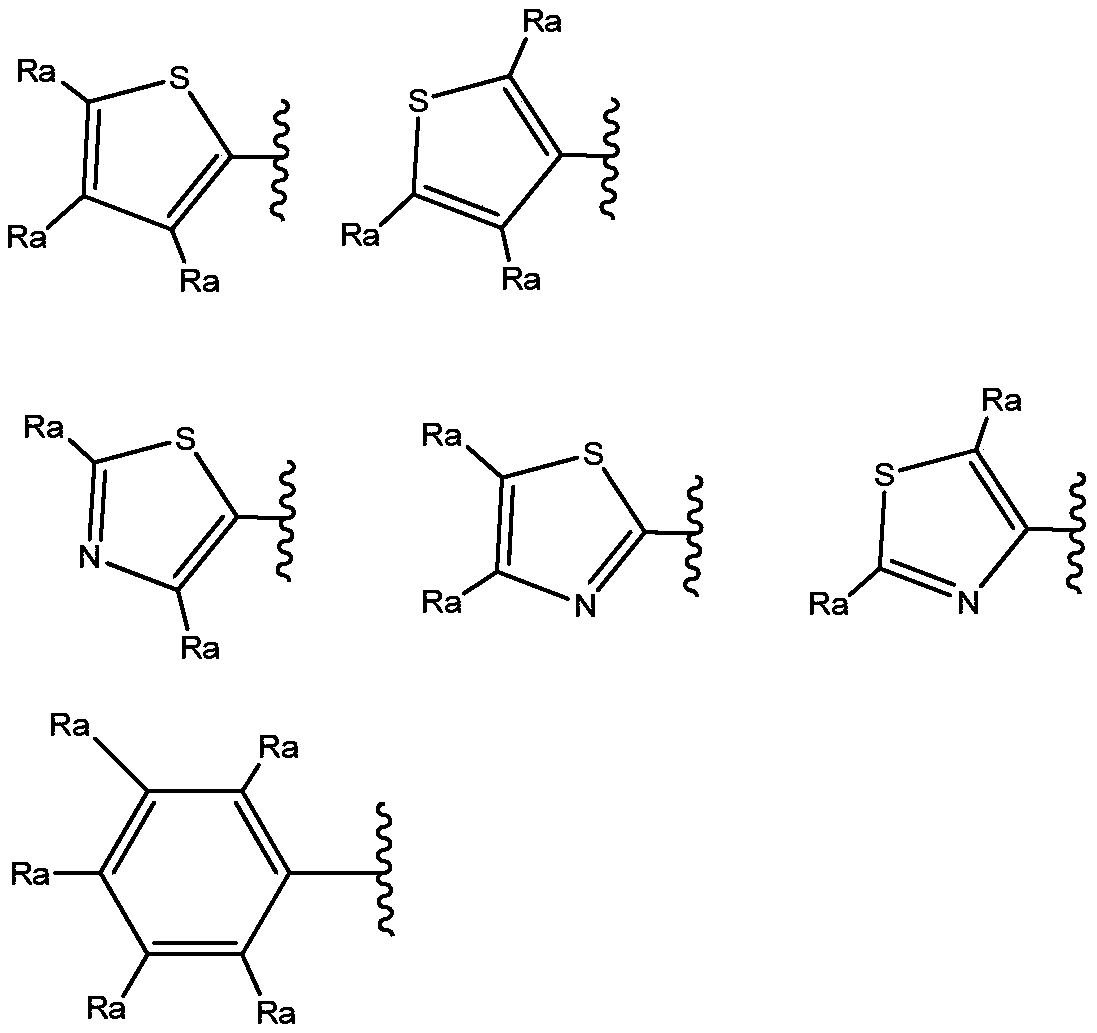
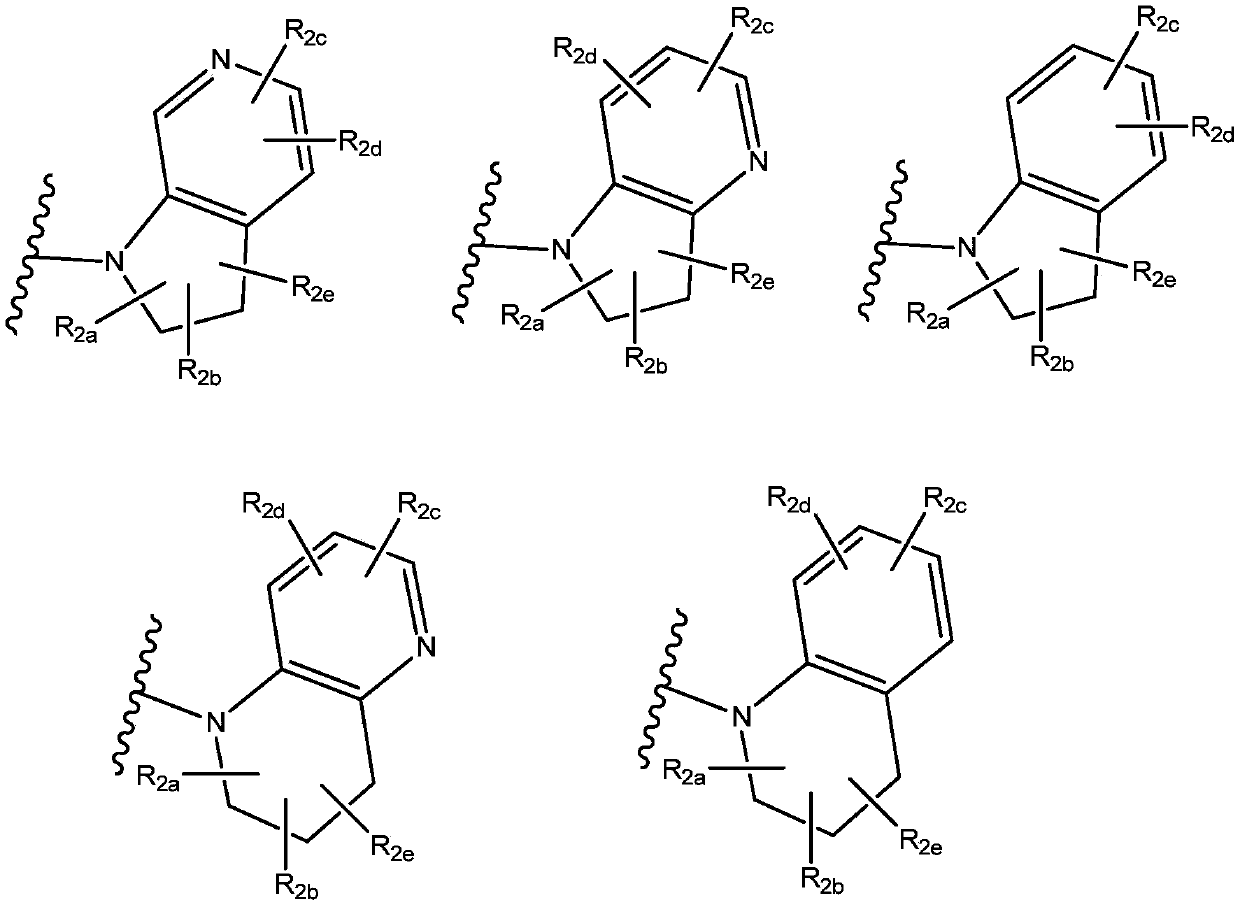
New propanamine derivatives for treating pain and pain related conditions
A compound, selected technology, applied in the direction of drug combination, neurological diseases, non-central analgesics, etc., can solve drug resistance and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0585] Example 1: 3-(indol-1-yl)-N-methyl-3-(thiophen-2-yl)propan-1-amine
[0586]
[0587] Step 1 1-(3-Chloro-(thiophen-2-yl)propyl)indoline: To a solution of indoline (92 mg, 0.77 mmol) in ACN (0.5 mL) was added K 2 CO 3 (53 mg, 0.38 mmol), and the mixture was stirred at room temperature for 30 minutes. Then, a solution of Intermediate 6 (50 mg, 0.26 mmol) in ACN (0.5 mL) was added dropwise, and the mixture was heated at 70 °C overnight. It was then allowed to cool and diluted with saturated ammonium chloride solution and EtOAc. The phases were separated and the aqueous phase was extracted with EtOAc. The combined organic phases were washed with brine, MgSO 4 Dry and concentrate to dryness. The crude product was purified by flash chromatography, silica gel, a gradient of CH / EtOAc 100:0 to CH / EtOAc 50:50 to afford the title compound (34 mg, 47% yield).
[0588] Step 2 Title compound: In a sealed tube, a solution of the product obtained in Step 1 (34 mg, 0.12 mmol) an...
Embodiment 2
[0590] Example 2: 3-(2,3-dihydro-1H-pyrrolo[3,2-c]pyridin-1-yl)-N-methyl-3-(thiophen-2-yl)propan-1- amine
[0591]
[0592]Step 1 3-(2,3-dihydro-1H-pyrrolo[3,2-c]pyridin-1-yl)-3-(thiophen-2-yl)propionic acid ethyl ester: at -78°C To a cooled solution of 2,3-dihydro-1H-pyrrolo[2,3-c]pyridine (157 mg, 1.31 mmol) in anhydrous THF (4 mL), LDA solution (1.5 M in THF / ethylbenzene / heptane, 1 mL, 1.5 mmol), and the mixture was stirred at -78°C for 30 minutes. Then, a solution of ethyl (E)-3-(thiophen-2-yl)acrylate (216 mg, 1.19 mmol) in anhydrous THF (4 mL) was added slowly, and the reaction mixture was stirred at -78°C for 1.5 hours. Join NH 4 Sat. aq. Cl and EtOAc, the phases were separated and the aqueous phase was extracted with EtOAc. The combined organic phases were washed with brine, MgSO 4 Dry and concentrate to dryness. The crude product was purified by flash chromatography, silica gel, gradient of CH / EtOAc 100:0 to CH / EtOAc 0:100 to afford the title compound (153 m...
preparation Embodiment 3-14
[0597]
[0598]
[0599] (1) Use ethylamine solution instead of methylamine in step 2
[0600] (2) Use ammonia instead of methylamine in step 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com