A kind of recombinant il-15 fusion protein and its application
A fusion protein and composition technology, applied in the field of medicine, can solve the problems of PEGylation of the target protein, high requirements, and decreased protein activity, so as to achieve the effect of preventing and treating tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Gene synthesis and vector construction of recombinant IL-15 fusion protein
[0046] IL-15 (SEQ ID NO: 1), IL-15Rα Sushi (SEQ ID NO: 2 ), the amino acid sequence of anti-HSA VHH (SEQ ID NO: 4) was assembled, and IL-15 and IL-15RαSushi were indirectly connected through a flexible linker peptide (SEQ ID NO: 3) to form a recombinant IL-15 fusion protein sequence ( SEQ ID NO:5), according to the codon optimization of human host cells and routinely synthesized genes, then add EcoRI restriction site and 5'UTR at the 5' end in sequence ([SEQ ID NO:6]); at the 3' end The 3'UTR ([TGATGA]) and HindIII restriction sites were added sequentially, and the gene was cloned into the vector pTT5 (SEQ ID NO: 7) via 5' EcoRI and 3' HindIII. Select the clones for sequencing, select the correctly sequenced bacteria for preservation and expand the culture of the bacteria, and the expanded bacteria are used for plasmid extraction.
Embodiment 2
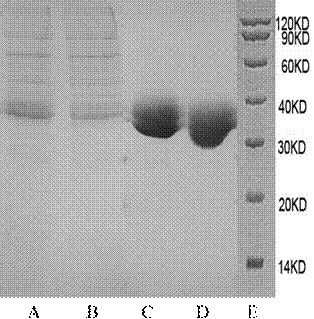
[0047] Example 2 Expression and purification of recombinant IL-15 fusion protein
[0048] Perform cell transfection and protein separation and purification on the extracted plasmids as follows:
[0049] 1. Measure the cell density. The viability should be greater than 95%. Use the preheated 293 medium to adjust the 293 cell density to 3×10 6 Cells / mL, shake gently and distribute the cells (transfection system 90%), the volume of cells in the shake flask should not exceed 1 / 3 of the shake flask specification, and put it in a shaker for use.
[0050] 2. Calculate the volume of transfection buffer opti-MEM according to the volume of transfected cells, which is 1 / 10 of the transfection system; calculate the amount of transfection reagent PEI, whose ratio is 3 μL / mL transfected cells; calculate the volume of transfected DNA The total amount, the ratio is 1 μg / mL transfected cells.
[0051] The specific transfection procedure is as follows:
[0052] Take a 50mL centrifuge tube, a...
Embodiment 3
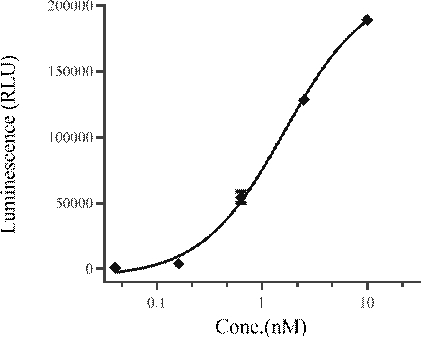
[0057] Example 3 Binding detection of recombinant IL-15 fusion protein and HSA
[0058] Using Gator TM A non-labeled bioanalyzer was used to detect the affinity of the recombinant IL-15 fusion protein expressed above and HSA protein (purchased from ACROBiosystems, catalog number HSA-H5220), and the Protein A biosensor was used to capture the antibody sample, and then the captured recombinant IL-15 15 The kinetic detection of the binding and dissociation of the fusion protein and the HSA protein. Kinetics were analyzed by fit using a 1:1 binding model. The brief steps are as follows: protein loading for 200s; binding for 180s; dissociation for 300s; regeneration for 30s.
[0059] Use Gator TM The affinity data determined by the instrument are shown in Table 1 below.
[0060] Table 1 Affinity test results of recombinant IL-15 fusion protein and HSA protein
[0061] antigen concentration fusion protein Response Affinity (M) Dissociation constant (1 / s) B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com