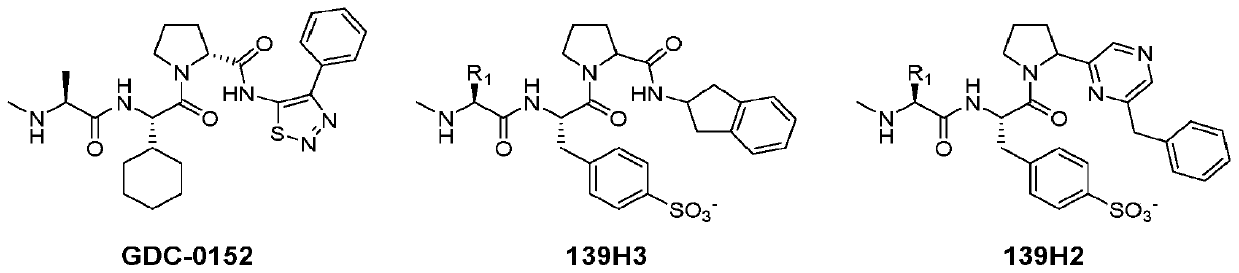
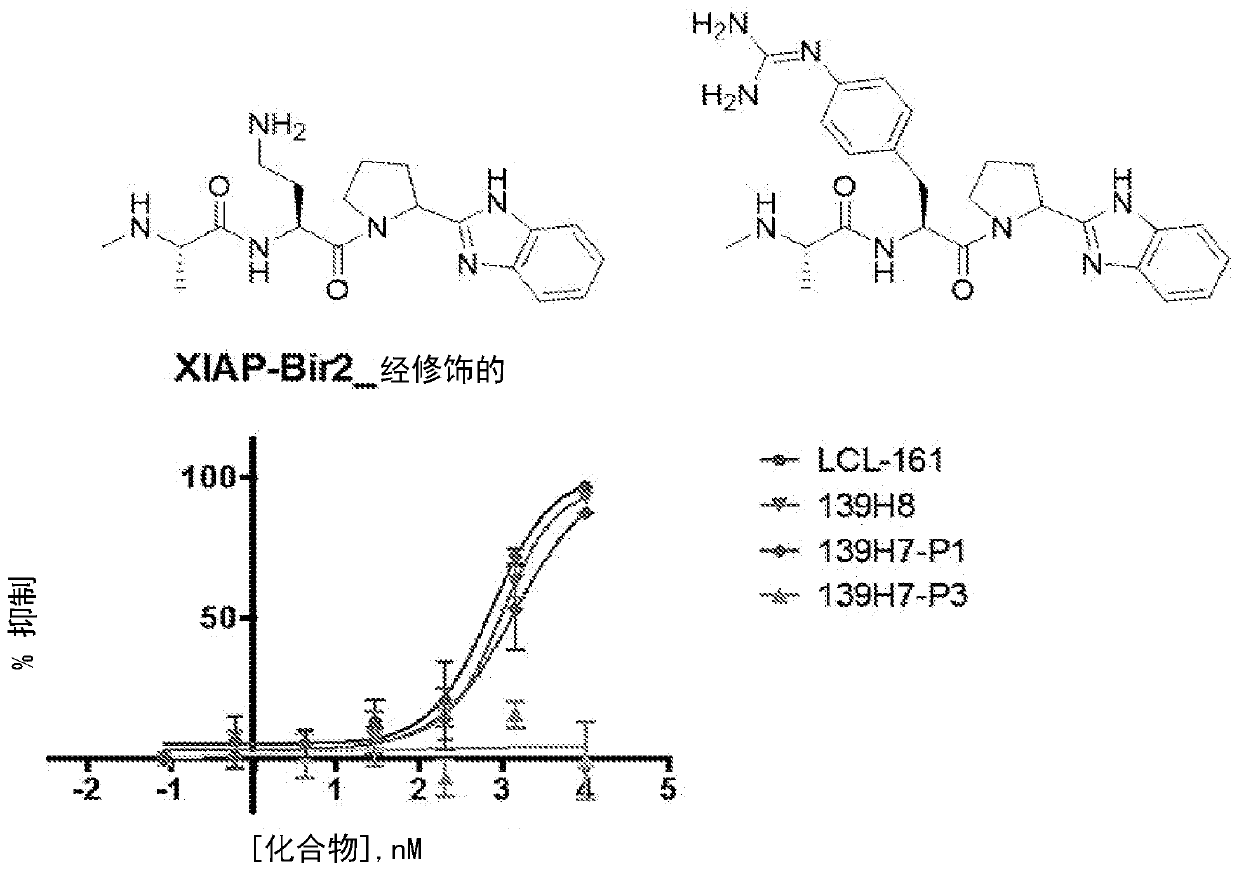
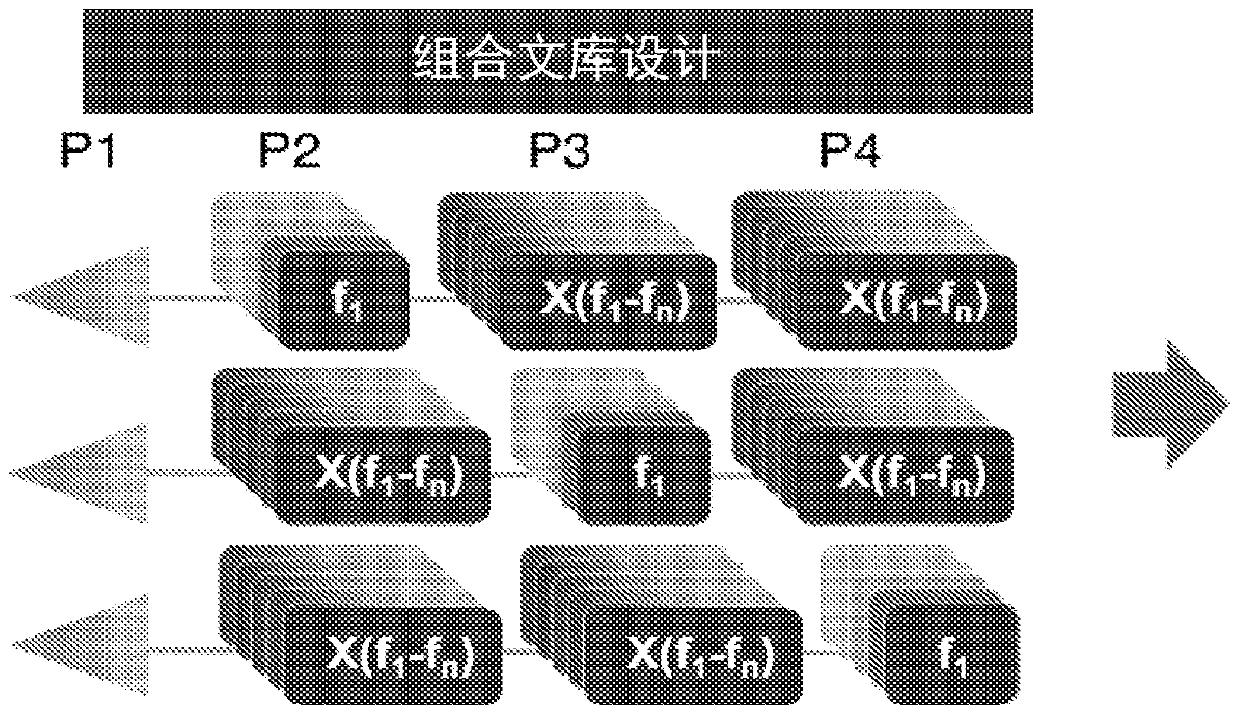
Novel agents targeting inhibitor of apoptosis proteins
A kind of technology of prodrugs, compounds, applied in the field of -3 and methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0563] Embodiment 1: According to an embodiment of the present invention, there is provided a compound having the general structure I listed below or a pharmaceutically acceptable salt thereof, including the form of a prodrug:
[0564]
[0565] Among them, R 1 is any of the following: -CH 3 、-C 2 h 5 、-CF 3 、-CH 2 F, -CHF 2 、-CH 2 CF 3 、-CF 2 CH 3 、-CH 2 OH, -CF 2 OH, -CHFOH; R 2 is the following group which is monosubstituted, disubstituted or trisubstituted: -CH 2 SO 3 - 、-PO 3 -2 、-SO 3 - 、-SO 2 NH 2 、-CH 2 PO 3 -2 、-CH 2 SO 2 NH 2 、-CF 3 , -Cl, -F, -CH 3 , -NO 2 、-C 2 h 5 、-OCH 3 、-OCF 3 , Guanidino, Acrylamide, -2-Chloroacetamide, -B(OH) 2 、-SO 2 F, -SO 2 CH=CH 2 , –COH, -CO-epoxide, -CO-aziridine; R 3 Represents monosubstitution, disubstitution or trisubstitution of the following groups: -F, -Cl, -CH 3 、-C 2 h 5 , -OH, -OCH 3 、-OCF 3 ;X,Y=C or N;R 4 and R 5 Can independently be –H, -F, -OH, -OCH 3 、-OCF 3 , Guanidine, -OC...
Embodiment approach 1-i
[0566] Embodiment 1-i: According to an embodiment of the present invention, there is provided a compound having the general structure I-i listed below or a pharmaceutically acceptable salt thereof, including the form of a prodrug:
[0567]
[0568] Among them, R 1 is any of the following: -CH 3 、-C 2 h 5 、-CF 3 、-CH 2 F, -CHF 2 、-CH 2 CF 3 、-CF 2 CH 3 、-CH 2 OH, -CF 2 OH, -CHFOH; R 2 is the following group which is monosubstituted, disubstituted or trisubstituted: -CH 2 SO 3 - 、-PO 3 -2 、-SO 3 - 、-SO 2 NH 2 、-CH 2 PO 3 -2 、-CH 2 SO 2 NH 2 、-CF 3 , -Cl, -F, -CH 3 , -NO 2 、-C 2 h 5 、-OCH 3 、-OCF 3 , Guanidino, Acrylamide, -2-Chloroacetamide, -B(OH) 2 、-SO 2 F. -OSO 2 F. -NHSO 2 F, -SO 2 CH=CH 2 , –NHSO 2 CH=CH 2 , –COH, -CO-epoxide, -CO-aziridine, epoxide, aziridine, oxaziridine; R 3 Represents monosubstitution, disubstitution or trisubstitution of the following groups: -F, -Cl, -CH 3 、-C 2 h 5 , -OH, -OCH 3 、-OCF 3 、-SO2 F, -SO ...
Embodiment approach 2
[0569] Embodiment 2: According to an embodiment of the present invention, there is provided a compound having the general formula II listed below or a pharmaceutically acceptable salt thereof, including the form of a prodrug:
[0570]
[0571] Among them, R 1 is any of the following: -CH 3 、-C 2 h 5 、-CF 3 、-CH 2 F, -CHF 2 、-CH 2 CF 3 、-CF 2 CH 3 、-CH 2 OH, -CF 2 OH, -CHFOH; R 2 is the following group which is monosubstituted, disubstituted or trisubstituted: -CH 2 SO 3 - 、-PO 3 -2 、-SO 3 - 、-SO 2 NH 2 、-CH 2 PO 3 -2 、-CH 2 SO 2 NH 2 、-CF 3 , -Cl, -F, -CH 3 , -NO 2 、-C 2 h 5 、-OCH 3 、-OCF 3 , Guanidino, Acrylamide, -2-Chloroacetamide, -B(OH) 2 、-SO 2 F, -SO 2 CH=CH 2 , –COH, -CO-epoxide, -CO-aziridine; R 3 Represents monosubstitution, disubstitution or trisubstitution of the following groups: -F, -Cl, -CH 3 、-C 2 h 5 , -OH, -OCH 3 、-OCF 3 ;X,Y=C or N;R 4 and R 5 Can independently be –H, -F, -OH, -OCH 3 、-OCF 3 , Guanidine, -OC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com