Application of benzamide compound in IAP protein inhibitor and in preparation of anti-tumor drug
A protein inhibitor, benzamide technology, applied in the field of biomedicine, can solve the problems of drug resistance, expensive targeted therapy, lack of targeted drugs, etc., to achieve antigenic response, good antitumor activity, and difficult activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
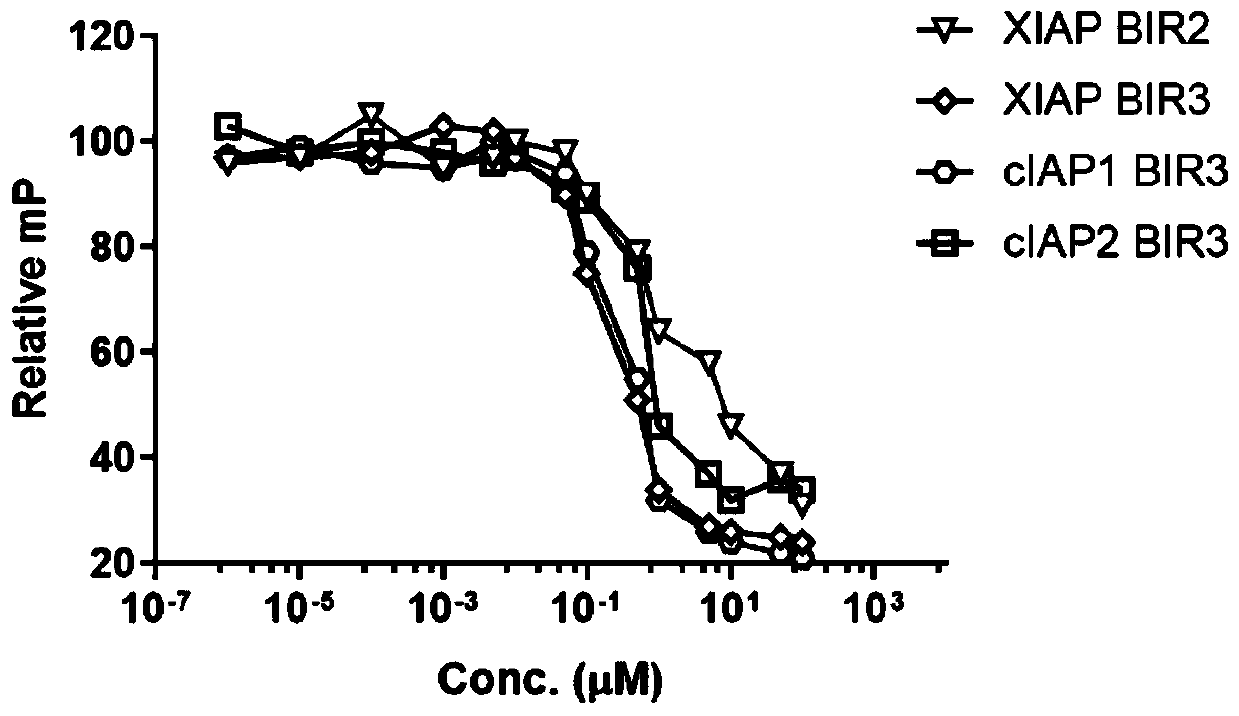
[0055] Research on the combination of benzamide compounds of the present invention and IAP protein.
[0056] 1. Molecular docking
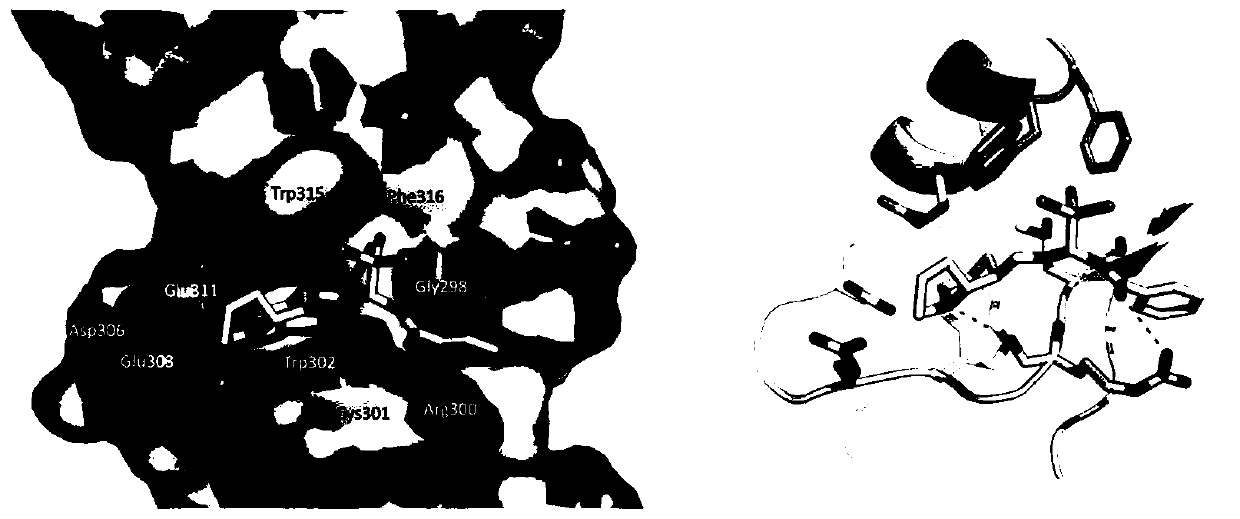
[0057] AutoDock Vina software was used for molecular simulation experiments; the structure of benzamide compounds was exported to pdbqt format using AutoDockTools; c-IAP1 and XIAP BIR3 domain crystal structures ( PDB ID: 3UW4) Download from the PDB database; remove all water and solvent molecules; add Gasteiger-Marsili potential to the protein structure, set the docking box size to Coordinates [x, y, z=26.375, -20.547, -47.589], other parameters are set to default; the results are exported from the PyMol online system (www.pymol.org); the exported results are as follows figure 1 as shown, figure 1 Middle: The red area represents the negative potential; the blue represents the positive potential, and the amino acid residues interacting with the benzamide molecules have been marked in the figure, and the dotted line represents the hydrogen bond;...
Embodiment 2
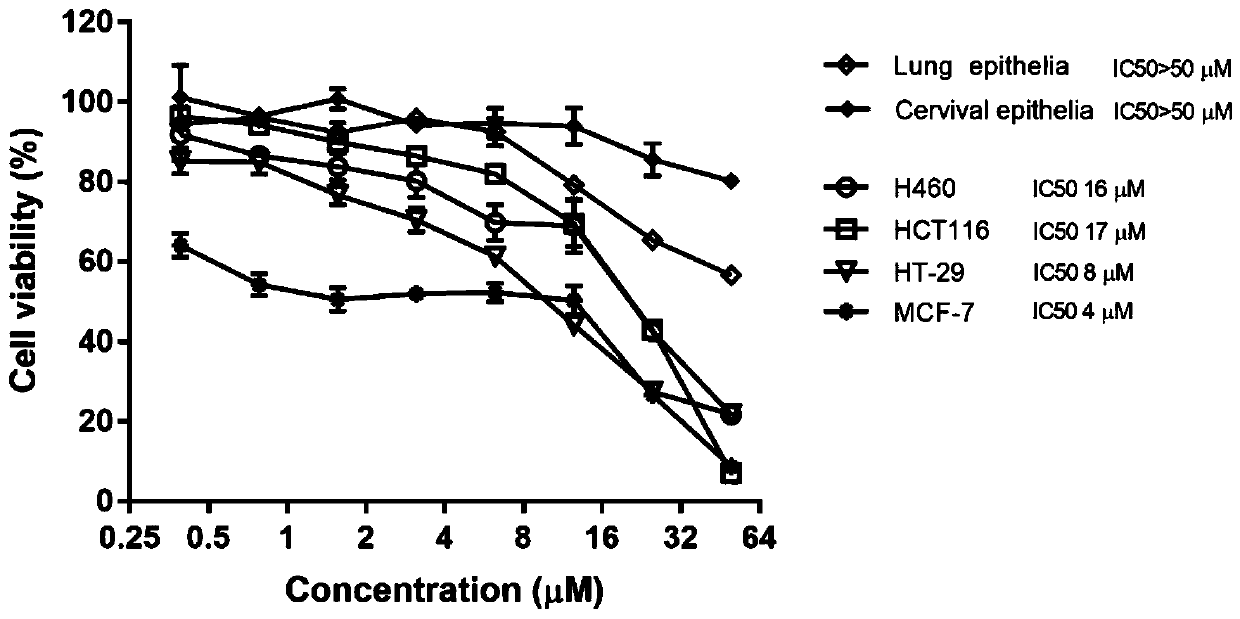
[0063] The benzamide compound of the invention significantly inhibits the growth of human lung cancer cells, human colorectal cancer cells and human breast cancer cells.
[0064] MTT experiment:
[0065] Human tumor cell lines (including lung cancer H460, colorectal cancer HCT116 and HT29, breast cancer MCF-7) were incubated at 37°C, 5% CO 2 , cultured and passaged in RPMI 1640 complete medium (containing inactivated fetal bovine serum 10%, streptomycin 100 μg / mL, penicillin 100 U / mL) under the condition of relative saturated humidity 95%; normal human epithelial cells (lung, cervical ) in conditioned medium (containing F12 basal medium 25%, hydrocortisone 25ng / mL, EGF 0.125ng / mL, amphotericin B 250ng / mL, gentamicin 10μg / mL, cholera toxin 0.1nM, insulin 5 μg / mL, ROCK inhibitor Y-276324 10 μM, and the balance is cultured in DMEM complete medium);
[0066] Take each cell grown in the logarithmic phase, press 3×10 3 The density of cells / well was inoculated in 96-well plates, a...
Embodiment 3
[0069] The benzamide compound of the present invention can significantly induce the apoptosis of human breast cancer MCF-7 cells.
[0070] Cell apoptosis was detected by flow cytometry:
[0071] Take the human breast cancer MCF-7 cells in logarithmic phase growth, press 2×10 5 Cells were seeded in 6-well plates at a density of one per well, and the cells adhered overnight; prepared RPMI1640 complete medium containing 0, 5, 10, 15, and 20 μM of benzamide compounds, and added them to corresponding well plates; 48 hours later, the cells were used After digestion with 0.25% trypsin, centrifuge to remove the supernatant, suspend with PBS, and centrifuge to remove the supernatant (the whole process is light, to reduce damage to the cells, and to remove the supernatant is to avoid absorbing the precipitate as much as possible to reduce the loss of cells); use Annexin V-FITC / PI kit was used to detect apoptosis; add 5 μL Annexin V-FITC to each sample and place in the dark for 10 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com