Preparation method of α-acetyl-γ-butyrolactone
A technology of butyrolactone and acetyl group, applied in the field of preparation of α-acetyl-γ-butyrolactone, can solve the problems of less than 85%, low yield and the like, and achieves reduction of hydrolysis, simple separation system, and elimination of containing Effect of Phosphorus Wastewater and Waste Salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
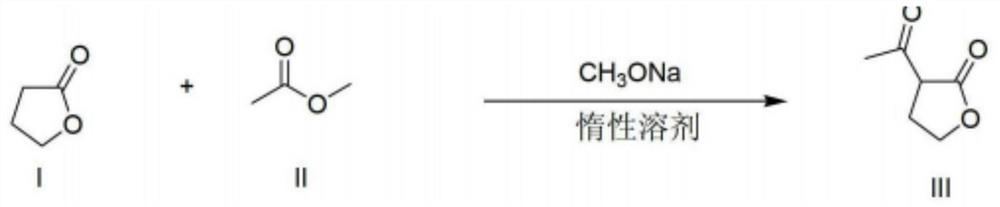
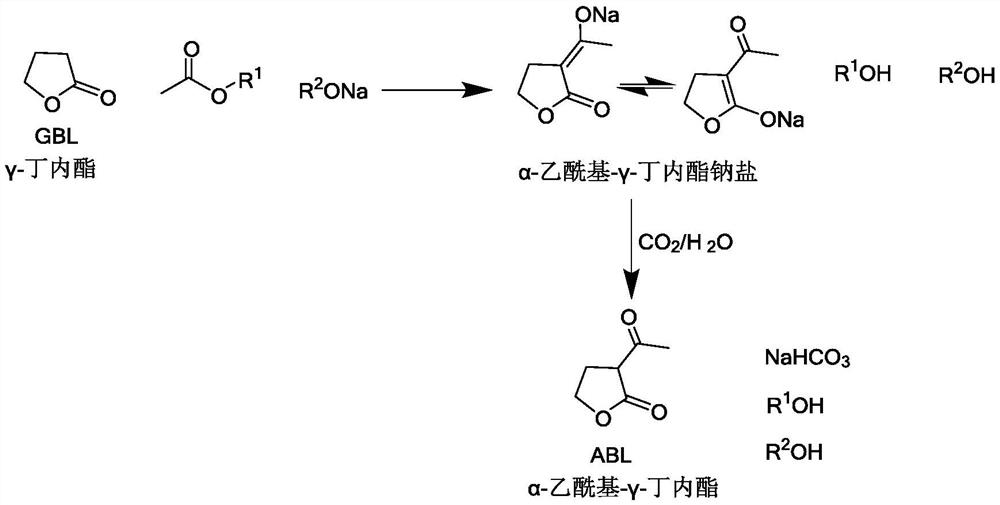
[0022] The invention provides a preparation method of α-acetyl-γ-butyrolactone, which comprises the following steps:
[0023] (1) Make γ-butyrolactone, CH 3 COOR 1 and R 2 Acylation of ONa to obtain a material containing α-acetyl-γ-butyrolactone sodium salt, wherein R 1 and R 2 each independently is a C1-C4 alkyl group;
[0024] (2) in the presence of water, make the material containing α-acetyl-γ-butyrolactone sodium salt and CO 2 The neutralization reaction occurs when the gas contacts.
[0025] According to the method of the present invention, preferably, in step (1), R 1 and R 2 Each independently is preferably a C1-C2 alkyl group such as methyl, ethyl. R 1 and R 2 Can be the same or different.
[0026] According to the method of the present invention, in step (1), the acylation reaction is generally carried out under dry conditions, and the acylation reaction is preferably carried out in a reaction tank equipped with a reflux and distillation device, and the re...
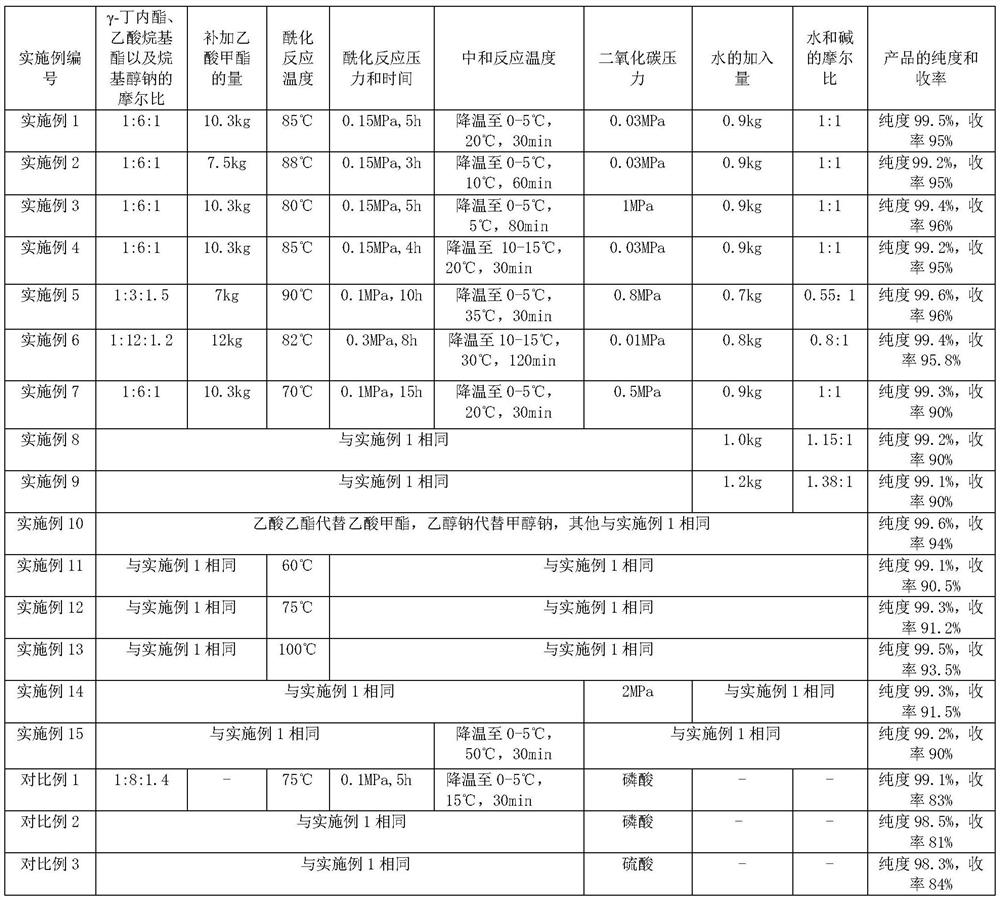
Embodiment 1
[0054] (1) Acylation reaction: The reaction was carried out in a dry 50L stainless steel reaction tank equipped with stirring, refluxing and distillation devices. Turn on stirring, replace the reaction tank with nitrogen, add 20.7kg of methyl acetate, raise the temperature in the reaction tank to 45°C, add 4kg of γ-butyrolactone and 2.6kg of sodium methoxide in batches, keep the system temperature at 43°C to between 48°C. The reaction system was gradually heated to 55°C, slowly opened the valve of the distillation unit, controlled the reflux ratio to be 4:1, and collected about 20 kg of the mixture of methanol and methyl acetate generated by the reaction, which was used for rectification, separation and recovery. At the same time, 10.3 kg of methyl acetate was slowly added, the temperature was slowly increased, the pressure in the tank was 0.15 MPa, and the reaction was maintained at 85 ° C for 5 h. About 1% of γ-butyrolactone remained in the reaction system by gas chromatogr...
Embodiment 2
[0059] (1) Acylation reaction: The reaction was carried out in a dry 50L stainless steel reaction tank equipped with stirring, refluxing and distillation devices. Turn on stirring, replace the reaction tank with nitrogen, add 20.7kg of methyl acetate, raise the temperature in the reaction tank to 45°C, add 4kg of γ-butyrolactone and 2.6kg of sodium methoxide in batches, keep the system temperature at 43°C to between 48°C. The reaction system was gradually heated to 55°C, slowly opened the valve of the distillation unit, controlled the reflux ratio to be 4:1, and collected about 20 kg of the mixture of methanol and methyl acetate generated by the reaction, which was used for rectification, separation and recovery. At the same time, 7.5 kg of methyl acetate was slowly added, the temperature was slowly increased, the pressure in the tank was 0.15 MPa, and the reaction was maintained at 88 °C for 3 hours. The reaction system was monitored by gas chromatography about 3% of γ-butyr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com