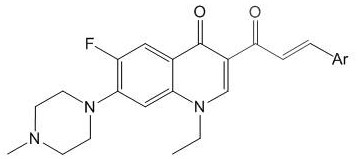
Acrylketone derivative of pefloxacin and preparation method and application of acrylketone derivative
A technology of pefloxacin and acrylone, which is applied in the field of drug synthesis, can solve problems such as the uncertainty of the effect of the C-3 carboxyl group of fluoroquinolones, and achieve the effects of increasing anti-tumor activity and drug resistance, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
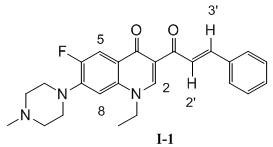
[0032] 1-Ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-3-cinnamoyl-quinoline-4(1 H )-ketone (I-1), its chemical structural formula is:
[0033]
[0034] That is, Ar in formula I is phenyl.
[0035] The preparation method of this compound is:
[0036] (1) Using pefloxacin shown in formula II as raw material, reacting with carbonyldiimidazole (CDI) to prepare norfloxacin imidazole amide compound shown in formula III, the specific preparation method is as follows:
[0037]
[0038] Take 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinoline-4(1 H 20 g (60.0 mmol) of )-keto-3-carboxylic acid II was dissolved in 500 mL of anhydrous acetonitrile, 15.2 g (94.0 mmol) of carbonyldiimidazole was added, and the mixed reactant was stirred and refluxed in a water bath until the raw material II disappeared. Leave it at room temperature, collect the resulting solid by filtration, and recrystallize with acetone to obtain a pale yellow crystal formula III with a yield of 83.5% and m.p. 232...
Embodiment 2
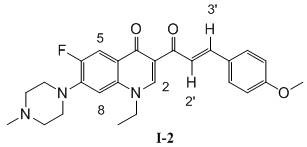
[0047] 1-Ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-3-(4-methoxycinnamoyl)-quinoline-4(1 H )-ketone (I-2), its chemical structural formula is:
[0048]
[0049] That is, Ar in formula I is p-methoxyphenyl.
[0050] The preparation method of this compound is:
[0051] (1) 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-1-yl-quinoline-4(1 H )-keto-3-ethanone V is prepared with reference to steps (1)-(3) of implementation 1, the solvent in step (1) is replaced by tetrahydrofuran solution, and the molar ratio of pefloxacin to carbonyldiimidazole is 1:1.0;
[0052] (2) Take 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinoline-4(1 H 1.0 g (3.0 mmol) of )-keto-3-ethanone V was dissolved in 20 mL of absolute ethanol, and 0.57 g (4.2 mmol) of 4-methoxybenzaldehyde and base catalyst piperidine (0.1 mL) were added. The mixed reactant was refluxed for 20 h, left at room temperature, and the resulting solid was collected by filtration and recrystallized from absolute ethanol to obtain ...
Embodiment 3
[0054] 1-Ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-3-(3,4-dioxymethylenecinnamoyl)-quinoline-4(1 H )-ketone (I-3), its chemical structural formula is:
[0055]
[0056] That is, Ar in formula I is 3,4-(dioxymethylene)phenyl.
[0057] The preparation method of this compound is:
[0058] (1) 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-1-yl-quinoline-4(1 H )-ketone-3-ethanone V is prepared with reference to steps (1)-(3) of Implementation 1, the solvent in step (1) is replaced by dioxane solution, the mixture of pefloxacin and carbonyldiimidazole The molar ratio is 1:2;
[0059] (2) Take 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-quinoline-4(1 H )-keto-3-ethanone V1.0 g (3.0 mmol) was dissolved in 20 mL of absolute ethanol, and 0.53 g (3.5 mmol) of 3,4-dioxymethylene benzaldehyde and base catalyst piperidine (0.1 mL). The mixed reactants were refluxed for 20 h, left at room temperature, and the resulting solid was collected by filtration and recrystallized from absolute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com