Oral formulations and uses thereof
A tablet and diluent technology, applied in their production, can solve the problems of changing the physical and chemical properties of the preparation, production problems, product instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0155] preformulation research
[0156] Characterization of Active Pharmaceutical Ingredient (API)
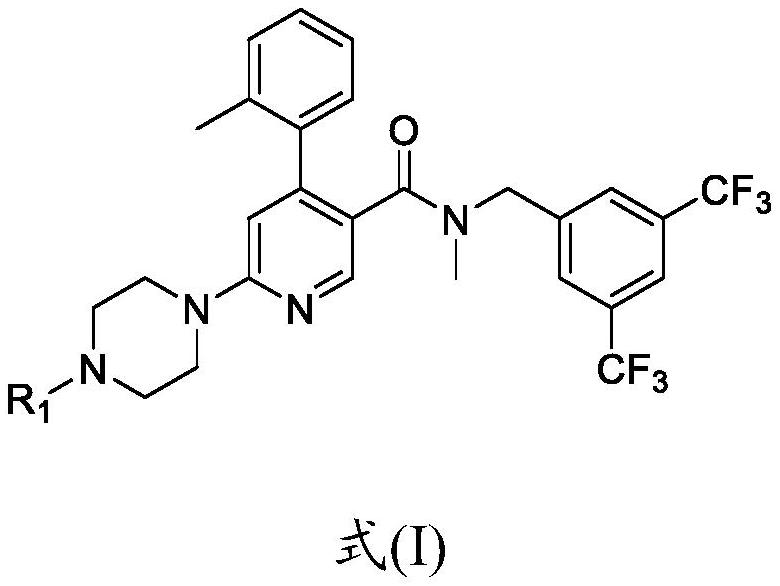
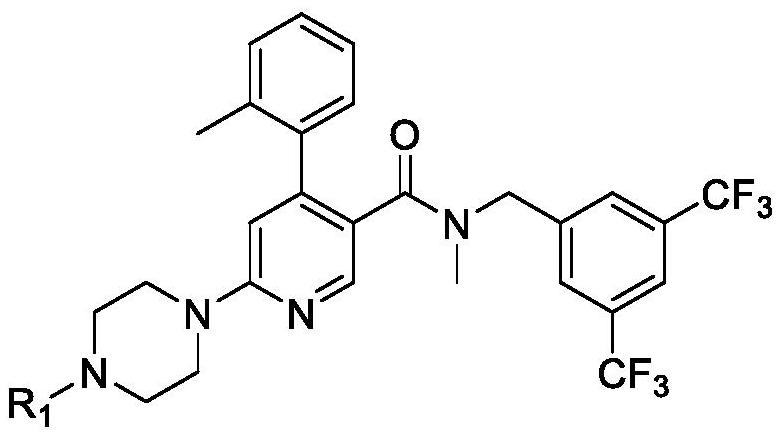
[0157] Compound (Ia) HCl(API) is an acidic compound with a pH of about 2.5. Compounds of formula (I), especially compound (Ia) shown below, especially the dihydrochloride salt of compound (Ia) (compound (Ia) 2HCl), were used in all examples.
[0158]
[0159] granularity
[0160] The particle size distribution of compound (Ia)2HCl(API) was determined using a Malvern Mastersizer. The results obtained are summarized in Table 1. D(0.1) means that 10% of the sample is present as particles smaller than this size. D(0.5) means that 50% of the sample is present in the form of particles smaller than this size. D(0.9) means that 90% of the sample is present in the form of particles smaller than this size.
[0161] Table 1: Particle size distribution of compound (Ia)
[0162]
[0163] bulk density and tap density
[0164] Measurements of bulk density and tap density ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com