Alkane chain or substituted alkane chain modified ruthenium complex and preparation method and application thereof
A ruthenium complex and alkane chain technology, which is applied in the field of alkane chain or substituted alkane chain modification of ruthenium complex and its preparation, can solve the problems of poor solubility, large toxic and side effects, etc., and achieves improved absorption capacity and improved transmembrane absorption capacity. , the effect of enhancing lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] The present invention provides a method for preparing the alkane chain or substituted alkane chain modified ruthenium complex described in the above scheme, comprising the following steps:
[0065] The ruthenium raw material, R 1 -Br, potassium carbonate and an organic solvent are mixed to carry out a substitution reaction to obtain an alkane chain or a substituted alkane chain modified ruthenium complex having a structure shown in formula I;
[0066] Wherein, the ruthenium raw material has a structure shown in formula II:
[0067]
[0068] In the present invention, in the ruthenium raw material having the structure shown in formula II, L, R 2 , R 3 and R 4 It is preferably consistent with the corresponding group in formula I, R 1 -R in Br 1 Preferably, it is consistent with the corresponding group in formula I, and will not be repeated here.
[0069] In the present invention, the source of the ruthenium raw material is not particularly limited, and can be obta...
Embodiment 1
[0089] Prepare ruthenium raw material, comprise the following steps:
[0090]
[0091] [RuL 2 Cl 2 ]·2H 2 O (105mg, 0.2mmol, wherein, L is o-phenanthroline group), ligand (0.4mmol, wherein, R 2 for-CF 3 , R 3 and R 4 is hydrogen), a mixed solvent of ethylene glycol and water (V 乙二醇 :V 水 =9:1), join in the three-necked bottle, and heat the oil bath to reflux at 120°C for 6h;
[0092] After the reaction, cool to room temperature (25°C), dilute the obtained system with water, filter, take the filtrate and add excess sodium persulfate to produce an orange solid, filter with suction, and dry the filter cake to obtain a crude product; the crude product is dissolved in acetonitrile and removed by filtration. Insoluble ligand, filtrate carries out neutral alumina column chromatography, with the mixed solution of acetonitrile and toluene (V 乙腈 :V 甲苯 =2:1) was used as the eluent for rinsing, and the eluate of the first band was collected; spin-dried, dissolved in acetonit...
Embodiment 2
[0098] The ruthenium raw material is prepared according to the method of Example 1, the difference is that in the ligand used, R 3 for-CF 3 , R 2 and R 4 is hydrogen, and the obtained ruthenium raw material is recorded as compound RPL082;
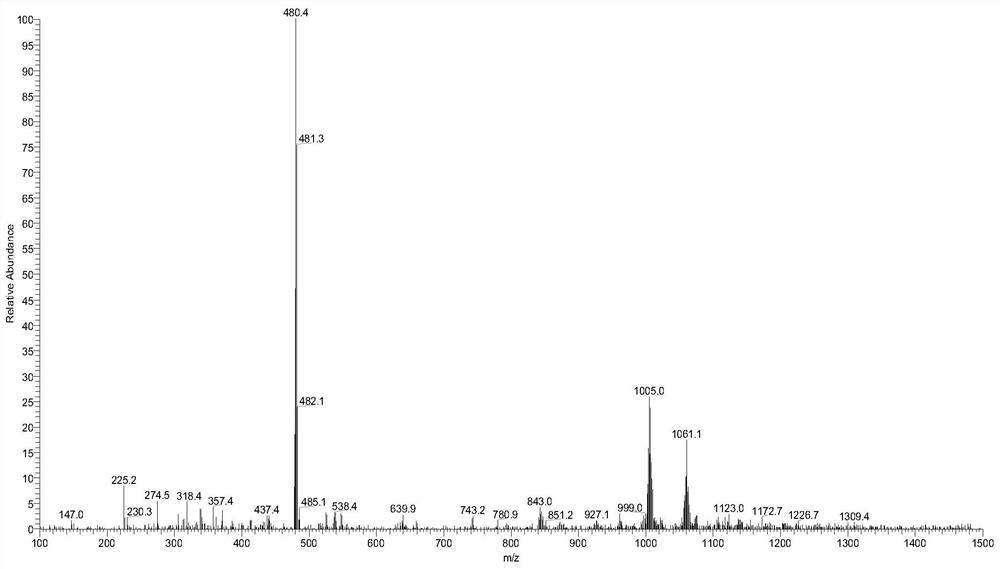
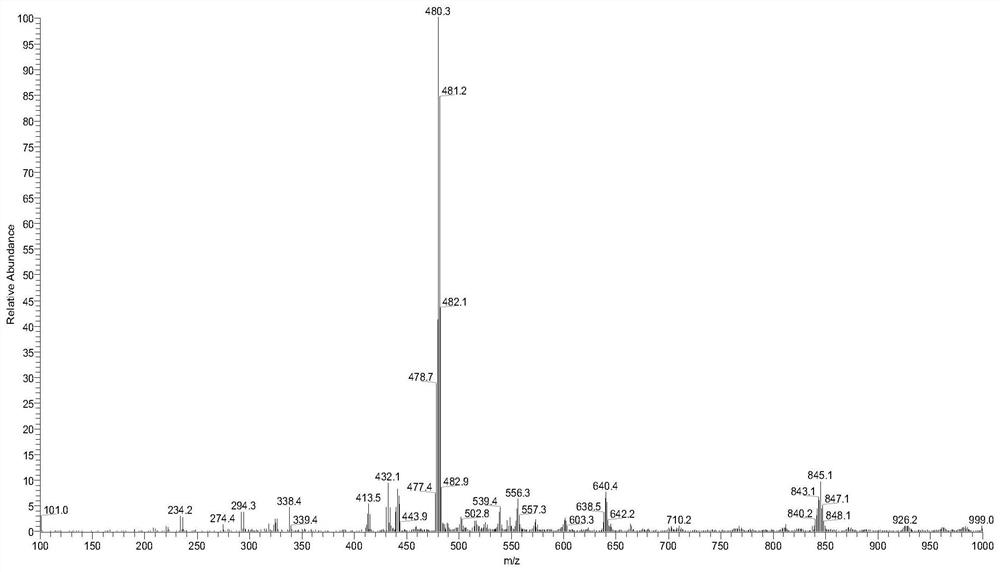
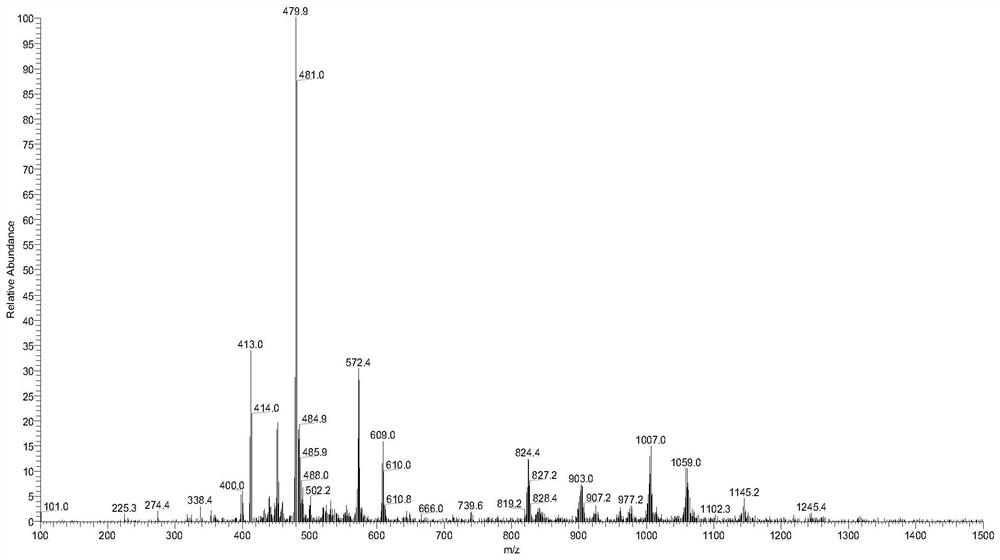
[0099] According to the method of Example 1, compound RPL082 is used to prepare a compound having the structure shown in formula 2, which is denoted as compound 2 (RPL082-BBr, and the ESI-MS spectrum is as follows figure 2 shown), the reaction scheme is as follows:
[0100]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap