Novel beta-glucosidase for producing glucose and laminarin oligosaccharide from seaweed
一种葡萄糖苷酶、昆布寡糖的技术,应用在新型β-葡萄糖苷酶领域,能够解决大能量、高热量等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
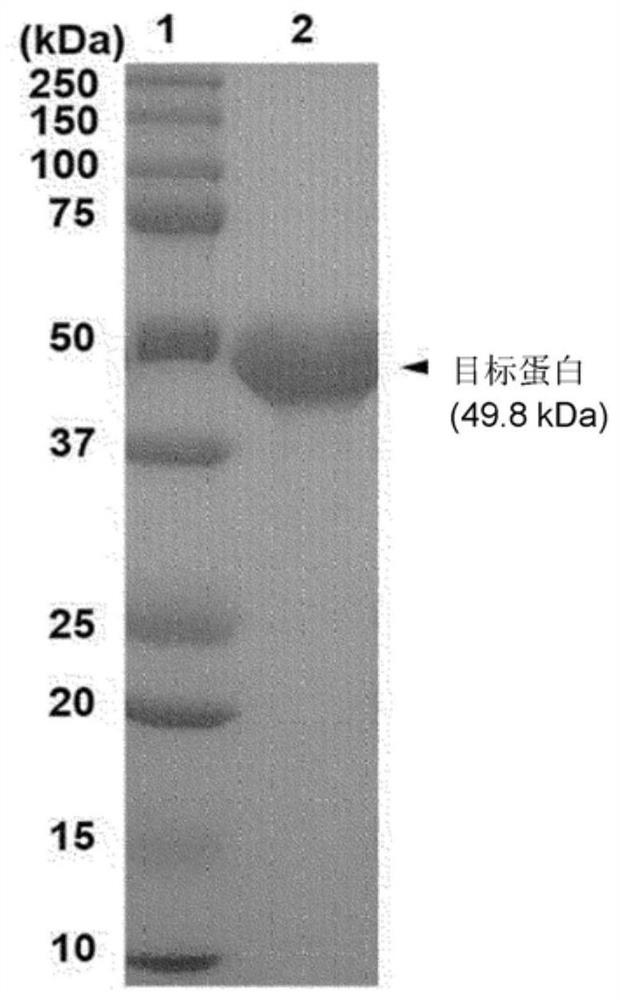
[0045] Overexpression and purification of Bgl1B
[0046] For obtaining proteus strains 2-40 T Genomic DNA (ATCC43961), the proteus strain 2-40 T They were cultured at 30° C. for 12 hours in a minimal medium containing 2.3 g of instant sea salt, 20 mM Tris-HCl, 2 g of glucose, 1 g of yeast extract, and 0.5 g of ammonium chloride per 1 L. Genomic DNA was extracted using a commercial DNA isolation kit (Qiagen, Valencia, CA, USA). The target gene bgl1B was amplified from genomic DNA by polymerase chain reaction (polymerase chain reaction, PCR). The primers used here were 5'-ATACATATGAATAGACTTACACTACCGCCTTCTTCTCGT-3' (forward: SEQ ID NO: 3) and 5'-ATAGCGGCCGCGCTCCTACTCGAGACAAACTCAGCAAATGC-3' (reverse: SEQ ID NO: 4). For protein purification by affinity chromatography, the nucleotide sequence of the gene encoding six histidine residues at the C-terminus was added. The PCR product and the pET21α vector were digested with NdeI and NotI, respectively, and ligated to construct the ...
example 2
[0049] Confirmation of the optimal pH and activation temperature of Bgl1B protein
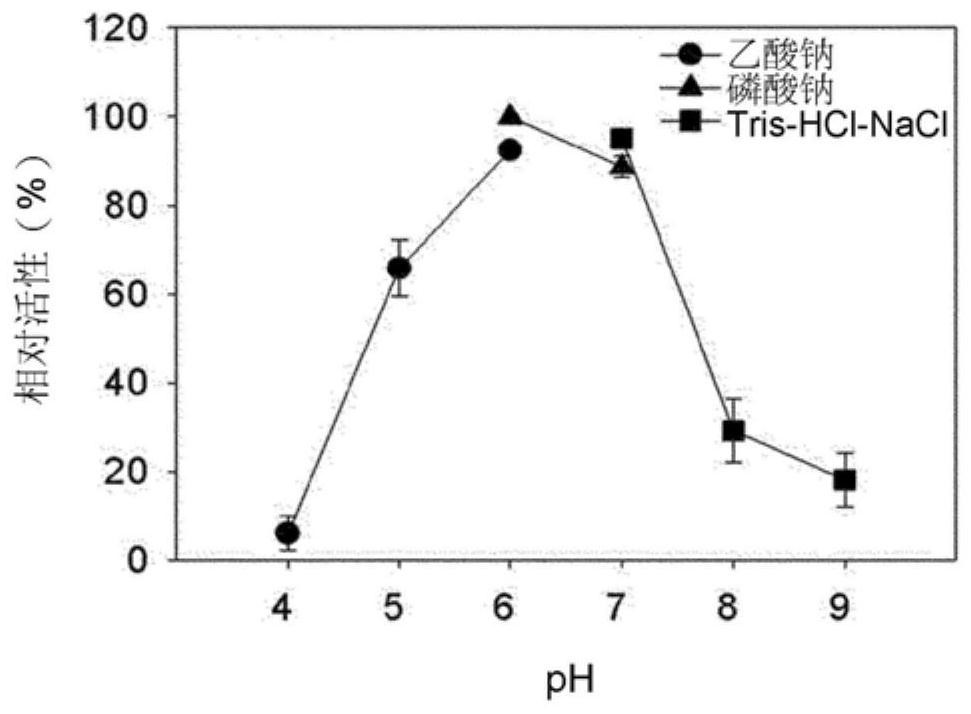
[0050] In order to explore the optimal active pH of Bgl1B protein, 20mM sodium acetate (pH 4.0 to pH 6.0), 20mM sodium phosphate (pH 6.0 to pH 7.0) and 20mM Tris-HCl-NaCl (pH 7.0 to pH 9.0) buffer (containing 0.1% (w / v) cellobiose) was reacted with Bgl1B protein at 40°C for 15 minutes.
[0051] figure 2 The relative activity of Bgl1B at pH ranging from 4.0 to 9.0 is shown. Bgl1B is most active at pH 6. At pH 4 and pH 8 or higher, the activity decreased to 30% or below.
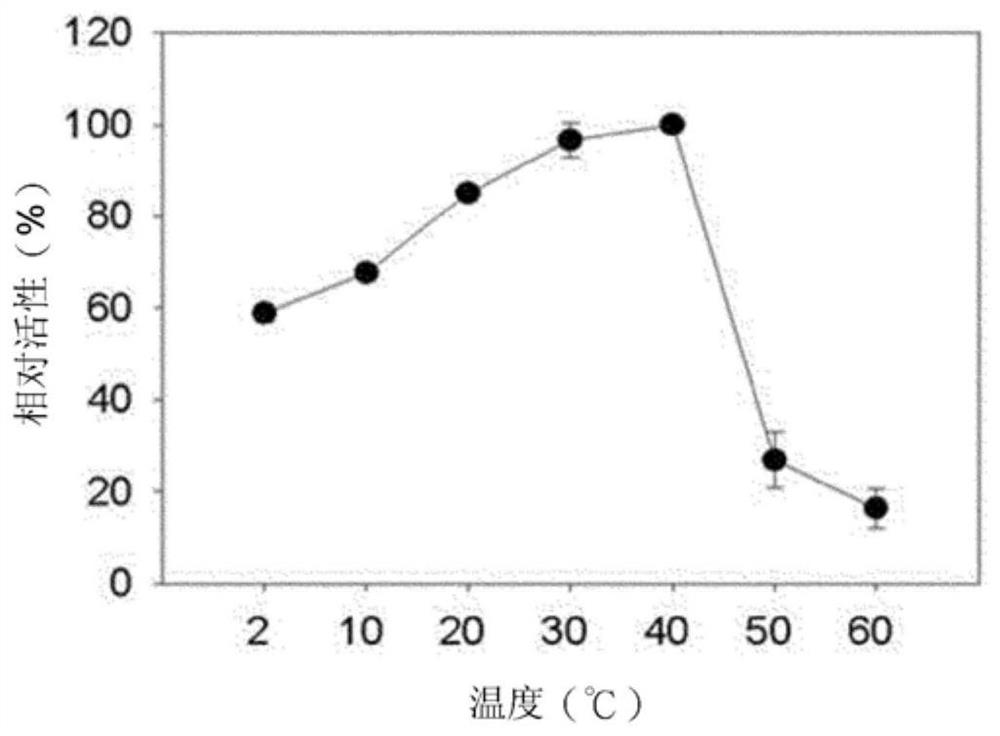
[0052] image 3 The relative activity of Bgl1B at temperatures ranging from 2°C to 60°C is shown. At pH 6, Bgl1B exhibited the highest enzymatic activity at 40 °C. At 20°C, about 83% of the enzyme activity is maintained. Furthermore, at very low temperatures (ie, 2°C to 10°C), an enzyme activity of 58% or higher was exhibited. However, at 50°C or higher, the enzyme activity sharply decreased. This indicates that the ...
example 3
[0054] Effect of metal ions on Bgl1B
[0055] In order to examine the effect of metal ions on the activity of Bgl1B protein, 10mM Mg 2+ , Ca 2+ , Mn 2+ 、Ni 2+ 、Cu 2+ , Fe 2 + and Co 2+ were added to 0.1% (w / v) cellobiose, and the relative activity of these cases was compared to that without metal ion addition.
[0056] As shown in Table 1, Mg 2+ 、Ni 2+ and Co 2+ Has no great effect on enzyme activity, but Mn 2+ Exhibited 56.2% enzyme activity. In addition, Cu 2+ and Fe 2+ A very low enzyme activity, ie 10%, was exhibited, indicating a very strong inhibitory effect.
[0057] [Table 1]
[0058] Metal ion Relative enzyme activity (%) control group 100.0±2.8 Mg 2+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com