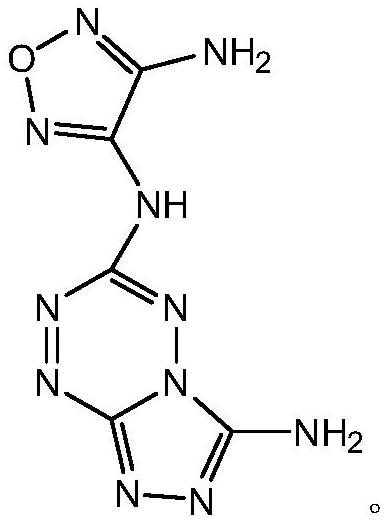
Tetrazine-furazan ring high-nitrogen energetic compound and synthesis method thereof
A technology with high nitrogen content and energy, synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of non-green environmental protection, long reaction time, unstable synthesis intermediates, etc., and achieve the effect of simple and safe operation and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
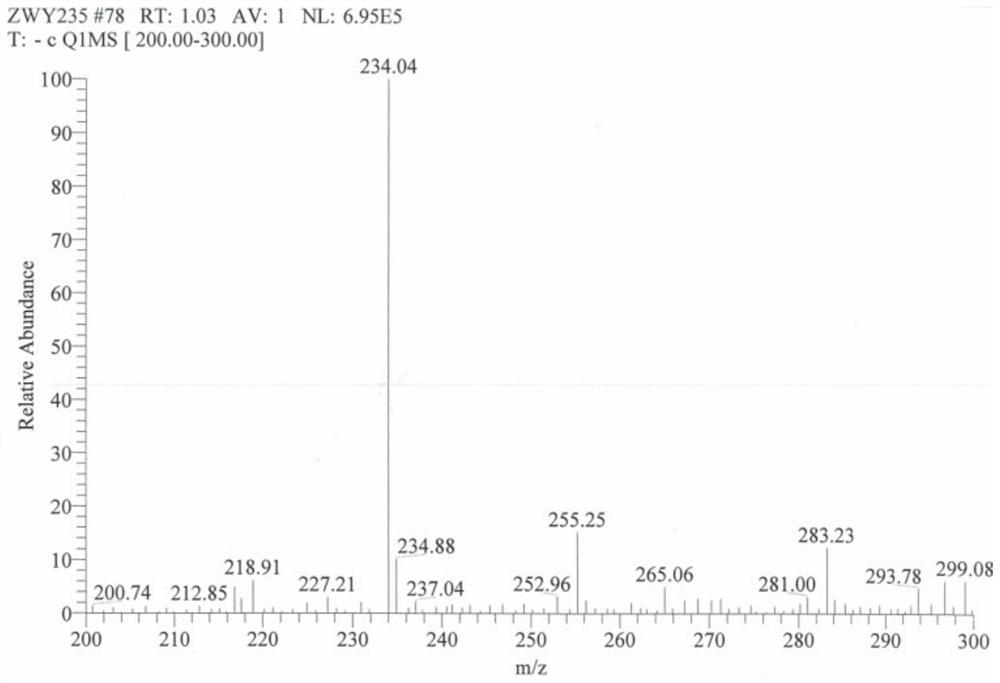
[0016] 1. Synthesis of tetrazine compounds through five-step reaction.
[0017] In the first step, add 80ML of distilled water, 200ML of 80% hydrazine hydrate, 42g of ammonium chloride, and 76.28g of guanidine hydrochloride into the reaction device, raise the temperature to 98°C, and react for 4 hours. After being placed in the refrigerator for 2 hours, it was suction filtered, washed with ice water, and dried in vacuo to obtain a white needle-like solid with a yield of 96%.
[0018]
[0019] In the second step, dissolve 50 g of the product from the previous step in 300 ML of distilled water, raise the temperature to 45 ° C, slowly add 61.7 mL of acetylacetone dropwise, after the drop is complete, raise the temperature to 70 ° C, and react for 2 hours. Cool to room temperature, vacuum filter, wash with water, and vacuum dry to obtain a light yellow powder solid with a yield of 94%.
[0020]
[0021] In the third step, 2.7 g of the product from the previous step was diss...
Embodiment 2
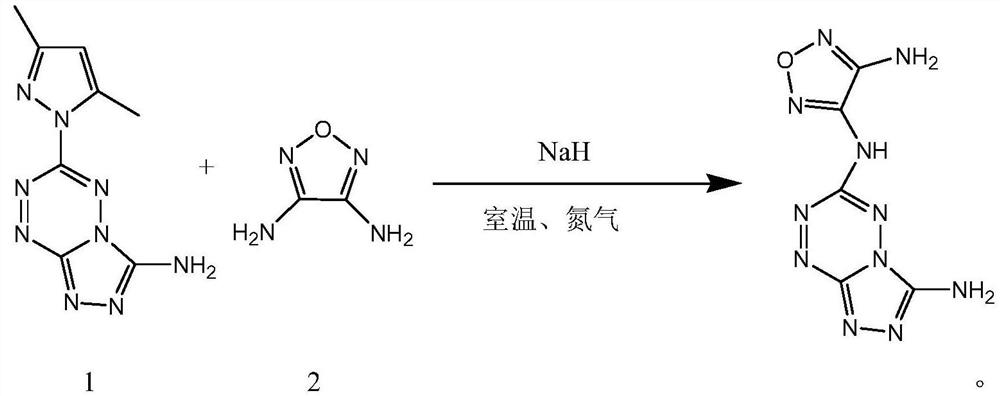
[0035] Other steps are the same as in Example 1. In the synthesis of the target compound, add 0.541g of 3,4-diaminofurazan and DMF25ML into a 50ML three-flask, under nitrogen protection at room temperature, stir until completely dissolved, then add 0.812g of NaH, and release a large amount of gas , stirred at room temperature for 15 minutes, 25 minutes, and 35 minutes. Then add 0.625 g of synthesized tetrazine compound and react for 40 minutes. As a result, the yields were found to be 50%, 81%, and 45%, respectively. The yield was highest when stirred for 25 minutes.
Embodiment 3
[0037] Other steps are the same as in Example 1. In the synthesis of the target compound, add 0.541g of 3,4-diaminofurazan and DMF25ML into a 50ML three-flask, under nitrogen protection at room temperature, stir until completely dissolved, then add 0.812g of NaH, and release a large amount of gas , and stirred for 25 minutes. Then add 0.625 g of synthesized tetrazine compound, react for 30 minutes, 40 minutes, and 50 minutes, and the yields are 54%, 81%, and 43%, respectively. The yield was highest at 40 minutes of reaction.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap