Short peptide inhibitor targeting calmodulin phosphatase and its substrate T-cell activation nuclear factor and its application
A calmodulin and phosphatase technology, applied in the field of short peptide inhibitors, can solve problems such as exhaustion, cardiovascular system lesions, aggravated hypertension, and hyperlipidemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
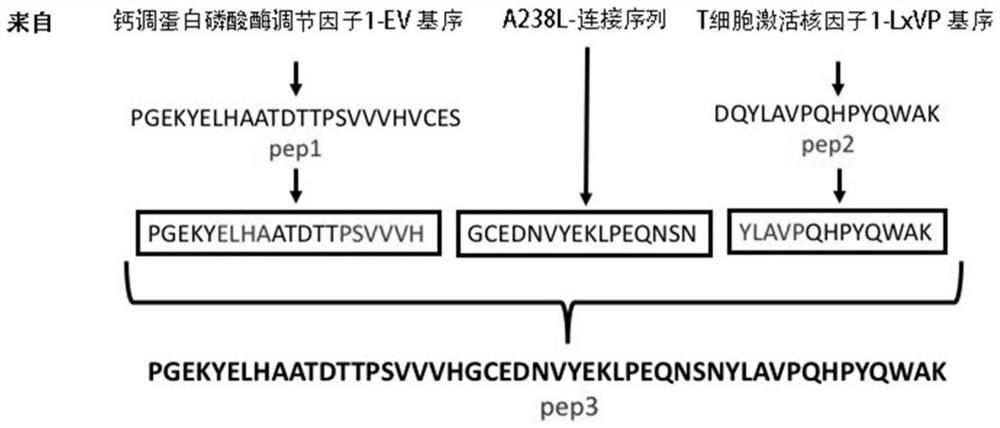
[0058] Example 1. Design of pep3 short peptide targeting calmodulin phosphatase and its substrate T cell activation nuclear factor and synthesis of gene sequence
[0059] The present invention aims at the binding site between CN and its substrate—PxIxIT site and LxVP site, and uses 17 amino acids of the short peptide A238L as Linker to link the EV motif (pep1) of RCAN1 with the LxVP motif (pep2) of NFATc1, An active polypeptide with two binding sites with CN was synthesized, named peptide3 (pep3 for short) ( figure 1 ). The amino acid sequences of the three pep short peptides and Linker peptides are shown in Table 1.
[0060] Table 1 Amino acid sequence of each short peptide involved in the present invention
[0061]
[0062] According to the codon preference of mammals, press figure 1 The amino acid sequence shown is a nucleic acid sequence synthesized at Huada Gene Biotechnology Co., Ltd. The nucleic acid sequence encoding the short peptide of Pep1 is shown in SEQ ID ...
Embodiment 2
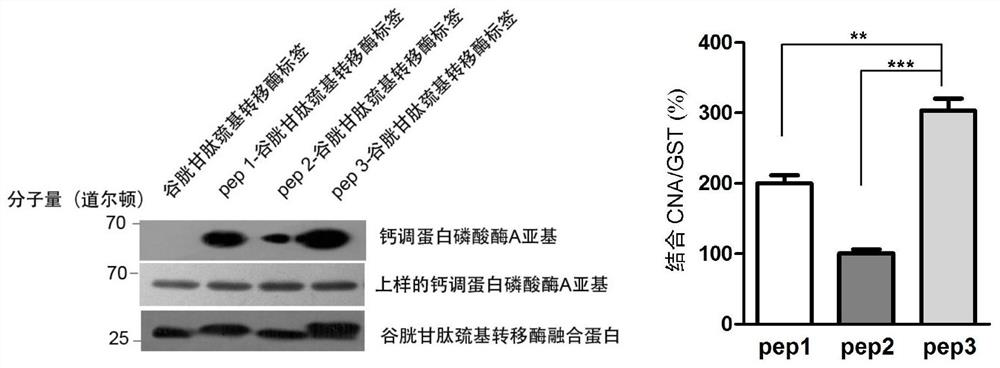
[0063] Example 2. The binding ability of pep3 short peptide to CN is much stronger than that of pep1 and pep2—GST Pull-Down experiment
[0064] 1. Construction of recombinant expression vector
[0065] The coding genes of the optimized Pep1, Pep2 and Pep3 short peptides in Example 1 were respectively cloned into the multiple cloning site of the pGEX-4T-1 vector fused to express the GST tag protein, so that the short peptide and the GST tag protein could be fused and expressed, Three recombinant expression vectors were obtained.
[0066] The correct recombinant expression vector for expressing Pep1 short peptide verified by sequencing was named pGEX-4T-1-Pep1; the correct recombinant expression vector for expressing Pep2 short peptide verified by sequencing was named pGEX-4T-1-Pep2; The correct recombinant expression vector for expressing Pep3 short peptide verified by sequencing was named pGEX-4T-1-Pep3.
[0067] 2. Transform Escherichia coli
[0068] The above-mentioned th...
Embodiment 3
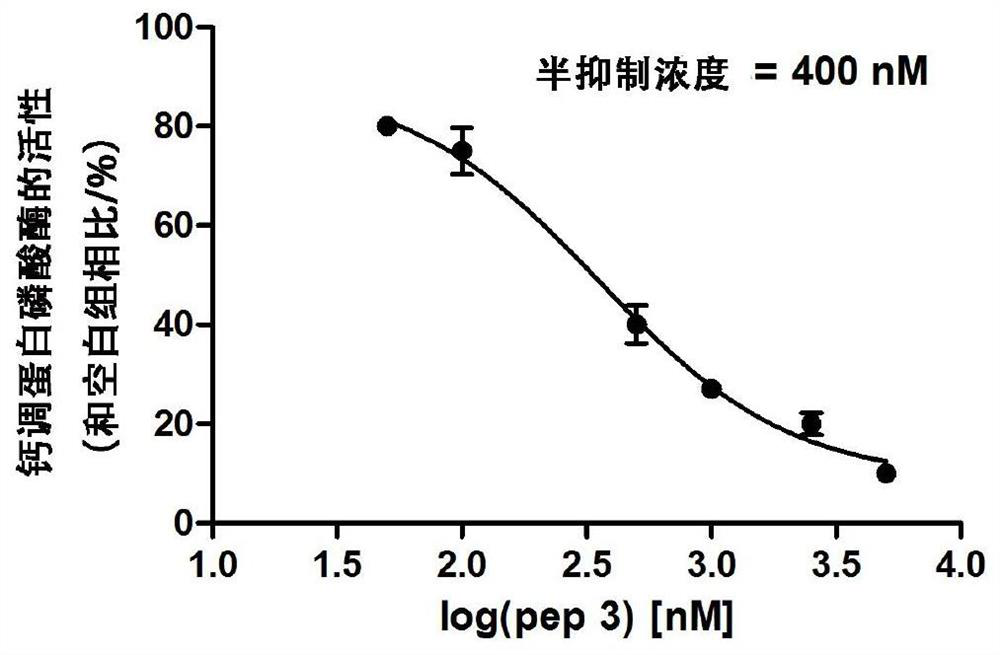
[0094] Example 3. Inhibition of CN activity by pep3 short peptide—using RII short peptide as a substrate to measure the activity of malachite green
[0095] The pep3 short peptide used in this implementation was synthesized by Zhongke Yaguang Biotechnology Co., Ltd., and its amino acid sequence is shown in SEQ ID No.4.
[0096] 1. Determination of proenzyme activity
[0097] The proenzyme activity of CN catalytic subunit (catalytic subunit, A subunit, CNA) on CN physiological substrate RII short peptide (RII subunit of protein phosphokinase, amino acid sequence is shown in Table 1) was determined. The experimental methods are shown in Table 2.
[0098] Table 2 CNA proenzyme activity assay scheme
[0099]
[0100] Note: The enzyme solution in the table is the original enzyme solution of CNA (enzyme solution with higher concentration of CN obtained by lysing the mouse brain). The enzyme diluent is the diluent (recipe: 50mM (pH=7.4) Tris-HCl; 0.1mM EDTA; 0.1mMEGTA; 0.2% (v / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com