Tumor neoantigen epitope peptide and its application
A tumor antigen and epitope peptide technology, applied in the field of molecular immunology, can solve the problems of unsatisfactory survival and achieve the effect of preventing recurrence and metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1 Prediction of Tumor Neoantigen Epitope Peptides
[0146] 1. Material preparation
[0147] The tumor tissues of tumor patients were obtained, and WES and RNA-seq sequencing of tumor tissues were completed through the illumina high-throughput sequencing platform.
[0148] 2. Data quality control
[0149] The original fastq data of DNA and RNA sequencing were quality controlled by FastQC software to obtain the data clean.fq.gz after quality control filtering.
[0150] 3. Data comparison
[0151] The DNA data after quality control was compared and analyzed using BWA software to obtain bam files of tumor and normal tissue DNA data, and the RNA after quality control was compared and analyzed using hisat2 software to obtain bam files of tumor RNA data.
[0152] 4. Bam file processing
[0153] The bam files after comparison need further processing. The bam files of DNA data are sorted by using samtools, Picard, GATK and other software to sort the bam files, remove...
Embodiment 2
[0171] Example 2 Detection of Neoplastic Antigen Epitope Peptide and Tetramer Binding Ability
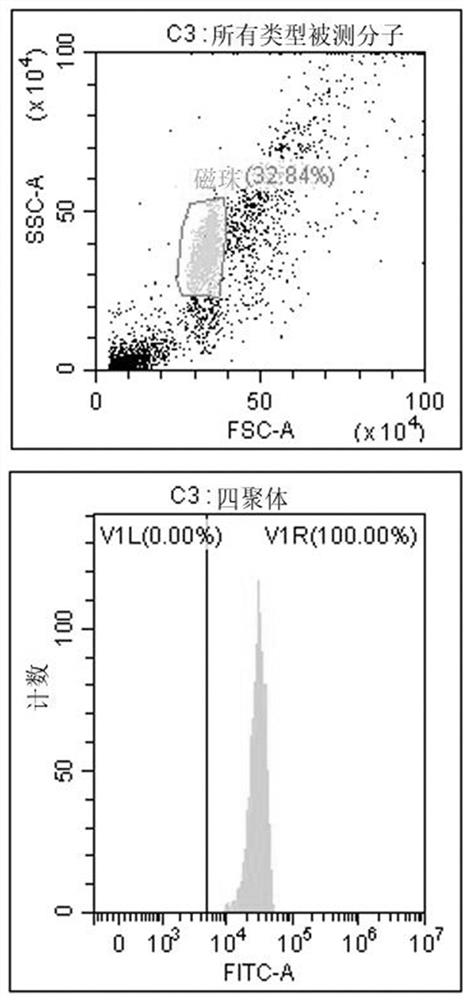
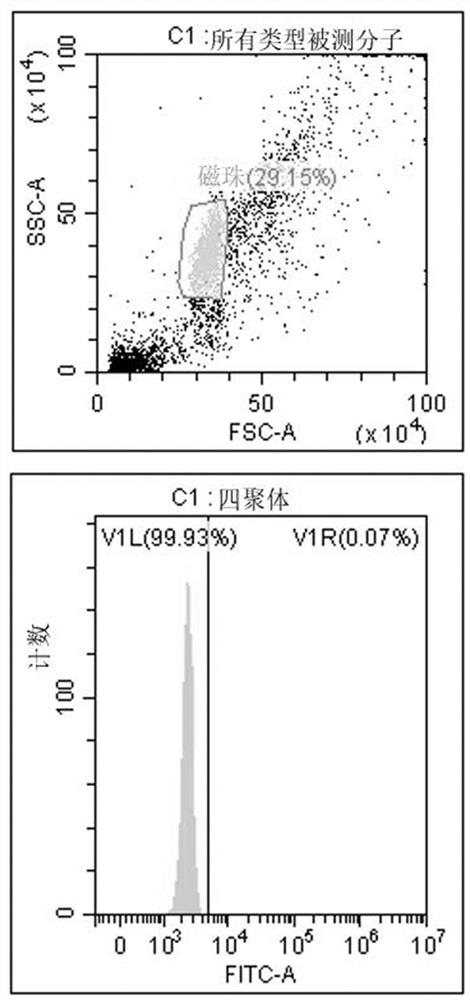
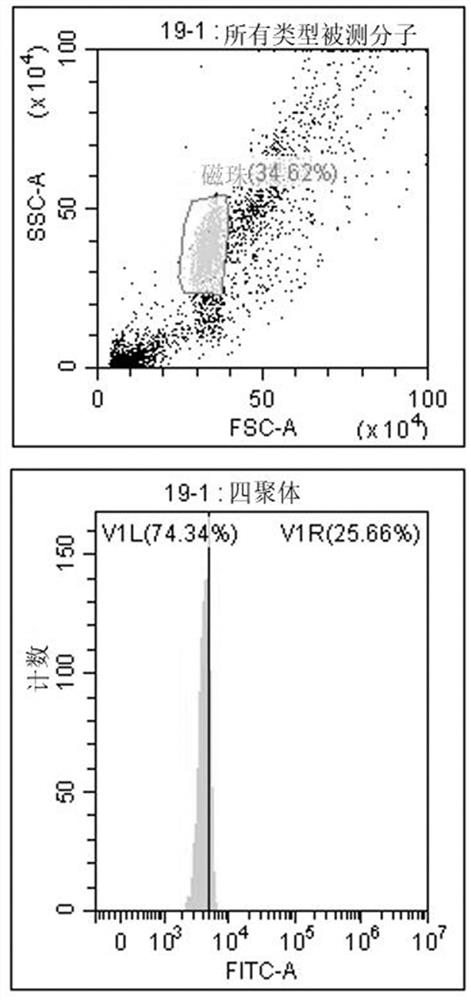
[0172] Tumor neoantigens to stimulate T cell activation must first be able to combine with HLA molecules of antigen-presenting cells (APCs) to form a complex expressed on the cell surface for recognition by T cell receptors on the surface of T cells. Therefore, recombinant tetramers were used to test the binding ability of predicted epitope peptides to tetramers.
[0173] The detection method is based on the ability of MHC class I molecules to replace peptides. MHC class I tetramers are formed from monomer units folded by a weak affinity peptide. Under the action of a suitable peptide replacement factor, it can produce Tetrameric complex that binds a specific peptide of interest. The detection technology is based on flow cytometry, and the main reagents include antibody-coupled magnetic beads that can capture MHC class I tetramers, and FITC-labeled antibodies that can recognize the...
Embodiment 3
[0186] Example 3. Analysis of cellular immune responses induced by tumor neoantigen epitope peptides
[0187] DC cells are loaded with neoantigen epitope peptides that can bind to HLA molecules and then injected into tumor patients. The frequency of antigen-specific T cells that produce γ-interferon is detected by ELISPOT experiment, and the tumor patients are analyzed after inoculation of neoantigen-loaded DC vaccines. Antigen-specific T lymphocyte responses.
[0188] 3.1 Elispot detection of specific cytotoxic T cells in peripheral blood of patient A
[0189] Whole blood was collected from patient A, and after the plasma was separated, human peripheral blood mononuclear cells (PBMCs) were isolated according to GE standard (GE Healthcare Life Sciences, Ficoll) operating procedures, and counted and viability were performed. Take a certain amount of PBMC to prepare a cell density of 2×10 6 / ml solution, then add the epitope peptide obtained in Example 2 into PBMC (the volume ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap